Question
Question: How many secondary carbon atoms are there in \({{\left( C{{H}_{3}} \right)}_{2}}CHC{{H}_{2}}CH{{\lef...
How many secondary carbon atoms are there in (CH3)2CHCH2CH(CH3)2?
Solution
A hydrocarbon chain may involve various carbon atoms. These are termed according to the carbon atoms attached adjacent to them. When a carbon has only one carbon atom attached, it is termed as primary carbon atom, while when it has 2 carbon atoms attached to it is called as secondary carbon atom. To recognize them a structural formula has to be drawn.
Complete answer:
A hydrocarbon long chain may have alkyl groups attached at various positions that define the nature of the attached carbon atoms as primary 1∘, secondary 2∘and tertiary 3∘. There are hydrogen atoms attached with these carbon atoms that are also termed in the same way as primary, secondary, and tertiary.
We have been given a compound that has a formula (CH3)2CHCH2CH(CH3)2, we have to identify the secondary carbon atoms. For this let’s see the structural formula and label the carbon atoms as, 1 to 5.
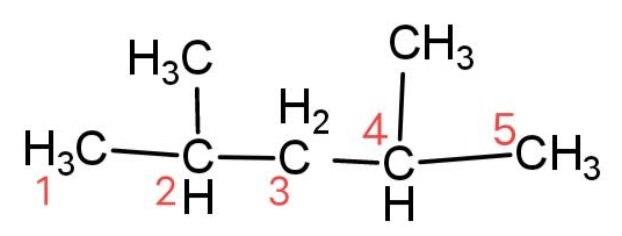
The secondary carbon atom is the carbon atom that is attached with two other adjacent carbon atoms. So the secondary carbon atom is carbon 3.
Hence, there is only 1 secondary carbon atom present in (CH3)2CHCH2CH(CH3)2.
Note:
The compound is called 2, 4 – dimethyl pentane. In this given compound there are 2 primary carbon atoms that are 1 and 5 carbons that are attached with only one carbon atom. Similarly there are 2 tertiary carbon atoms that are carbon 2 and carbon 4 that have 2 carbon atoms attached to them.
