Question
Question: How many same atoms of \(PC{l_3}{F_2}\) lie in the same plane?...
How many same atoms of PCl3F2 lie in the same plane?
Solution
We can find the number of atoms lying in the same plane by drawing its geometry with the help of hybridization, i.e. by finding the number of orbitals which the atoms lie. When the atoms lie in the same plane then it is said to be planar.
Complete step by step answer:
Let us start by finding the hybridization of the above structure. The formula used for hybridization is
X=21[V+M−C+A]
Where, V= number of valence electrons of the central atom.
M= number of monovalent atom
C= number of positive charge
A= number of negative charges.
In the case of PCl3F2, The central metal atom is P since it belongs to the nitrogen family so the number of valence electrons in P is 5. There are 3 monovalent chlorine atoms and 2 monovalent fluorine atoms. Hence the total monovalent atoms are 5 and there is no overall positive of negative charge on the compound. Hence the hybridization will be sp3d and it can be calculated as below:
X=21[5+5] ⇒X=21×10 ⇒X=5
Hence, its geometry will be trigonal bipyramidal and the structure is shown below:
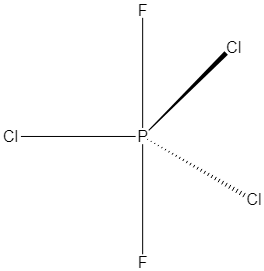
Here three chlorine atoms are in equatorial positions and two fluorine atoms are in axial position. So three same atoms of chlorine of PCl3F2 lie in the same plane.
Note:
The bond length of the axial bond is greater than the equatorial bond because the axial bond pair is repelled by three equatorial bond pairs whereas the equatorial bond pair is repelled by the axial bond pair. But the repulsion experienced by the axial bond pair is more.
