Question
Question: How many \(S-S\) linkage (s) is /are present in \({{H}_{2}}{{S}_{4}}{{O}_{6}}\)?...
How many S−S linkage (s) is /are present in H2S4O6?
Solution
Construct the structure of H2S4O6 by taking into consideration the valences of all the atoms and how they might be bonded to form a stable structure which is called tetrathionic acid.
Complete answer:
First, we will see what an S−S linkage actually is; it is the sigma bond found between 2 atoms of sulphur. Now, if we arrange the given atoms in H2S4O6 in the proper order, then we will get the number of S−S bonds present.
We know that the valency of sulphur is 6, oxygen is 2, and hydrogen is 1. Thus, by arranging the atoms such that all the valences are satisfied and we get a structure that can be called tetrathionic acid, we get:
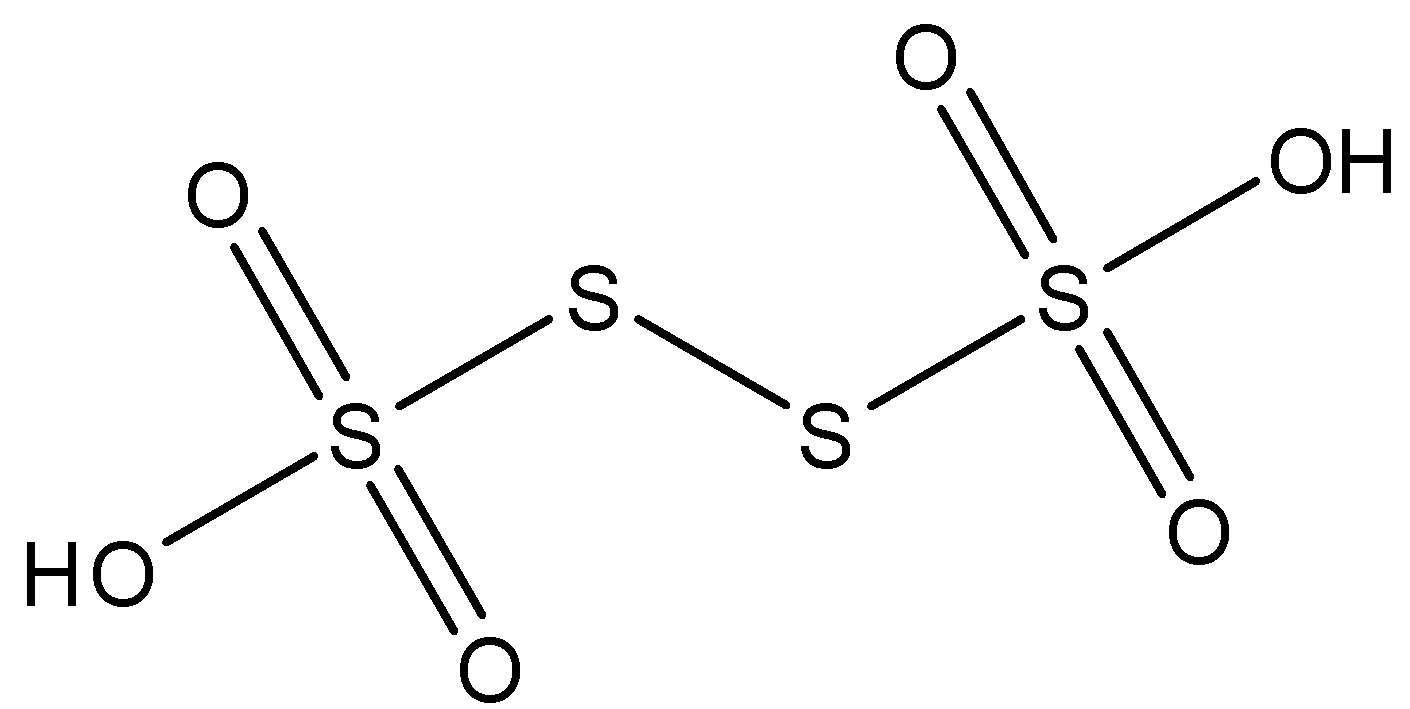
From the structure it is clear that H2S4O6 is an oxo acid, which contains straight chain of sulphur atoms. This can be depicted from the formula H2SnO6. We can also see the two acidic hydrogens in the hydroxyl groups that contribute to the name tetrathionic acid. We can clearly see that the number of S−S bonds present are 3, in the general formula of oxo acids the number of S−S bonds present will be (n−1).
Therefore, 3 S−S linkages are formed by H2S4O6.
Note:
The oxidation state of 2 sulphur atoms in tetrathionic acid is +5. The oxidation state of the 2 central sulphur atoms in tetrathionic acid is 0. Thus, not all the sulphur atoms have the same oxidation state and the average oxidation state of sulphur will come out to be a fraction if we calculate it using usual methods. You can make use of this to predict the structure.
