Question
Question: How many S – S bonds, S – O – S bonds, σ-bonds, and π-bonds are present in a trimer of sulphur triox...
How many S – S bonds, S – O – S bonds, σ-bonds, and π-bonds are present in a trimer of sulphur trioxide?
(A) 0, 3, 16, 2
(B) 0, 3, 12, 6
(C) 0, 6, 12, 16
(D) 0, 4, 12, 6
Solution
Hint: Sulphur trioxide forms a cyclic trimer by combining three SO3 molecules together. Both the types of bonds can be identified and the number of bonds can be calculated from the structure of the trimer.
Complete answer:
Sulphur trioxide is one of the most well-known chemical compounds that acts as a precursor to the sulfuric acid. Sulphur trioxide has a chemical formula of SO3. It can significantly be a primary component of acid rain.
The structure of SO3 molecule is given below:
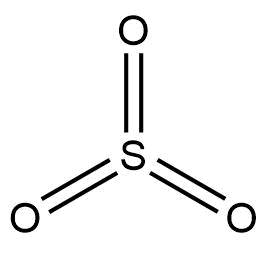
Now, sulphur trioxide can form a cyclic trimer where three SO3 molecules are connected together, or it can form a long chain of polymers as well.
In order to determine how many bonds are present and what types of bonds are present in a trimer molecule of sulphur trioxide, then we have to look at its structure. The structure of the trimer of sulphur trioxide is elaborated below:
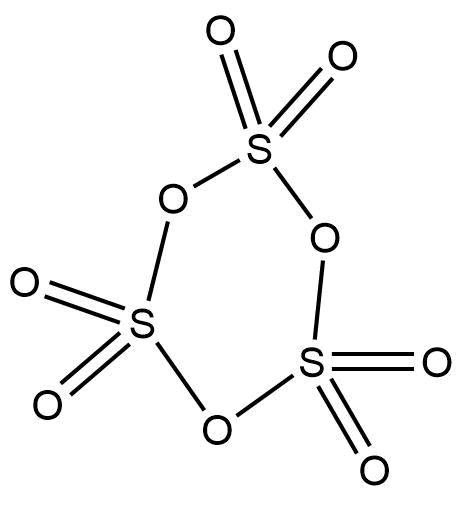
Now, let’s identify how many bonds are present that are mentioned in the question:
- S – S bonds: There is not a single S – S bond is present in the molecule (no two sulphur atoms are directly connected together). Hence, the number is 0.
- S – O – S bonds: There are 3 sulphur atoms in the trimer and each sulphur atom is connected to another sulphur atom via an oxygen atom. Thus, there are three S – O – S bonds. Hence, the number is 3.
- σ-bonds: Any single bond is considered to be a σ-bond. In a double bond, there is one σ-bond and one π-bond. In this molecule, there are 6 σ-bonds counted directly from the single bonds and another 6 σ-bonds that are counted from 6 π-bonds. Thus, there are a total of 12 σ-bonds. Hence, the number is 12.
- π-bonds: It is clearly seen that there are 6 double bonds in this trimer, which means there are 6 π-bonds in this molecule. Hence, the number is 6.
Now, let’s look at the answer options available:
A. 0, 3, 16, 2: The number of σ-bonds and the π-bonds are not 16 and 2 respectively. Hence, option A cannot be correct.
B. 0, 3, 12, 6: All the numbers match with our determination. Hence, option B is the potential answer.
C. 0, 6, 12, 16: The number of S – O – S bonds and π-bonds are not 6 and 16 respectively. Hence, option C cannot be correct.
D. 0, 4, 12, 6: The number of S – O – S bonds is not 4. Hence, option D cannot be correct.
Hence, option B is the correct answer to this question.
Note: To calculate the number of bonds correctly, students have to draw the structure of the trimer correctly. While calculating the σ-bonds, students have to take the double bonds into consideration, because in a double, one is σ-bond and other one is a π-bond.
