Question
Question: How many \(s{p^3}\) hybridised carbon atoms are in Acetophenone?...
How many sp3 hybridised carbon atoms are in Acetophenone?
Solution
Acetophenone is an organic compound. Acetophenone is the simplest aromatic ketone with the chemical formula C6H5C(O)CH3. sp3 hybridisation is formed by the intermixing of one s orbital and three p orbitals.
Complete step by step solution
The concept of hybridisation is given by Pauling. According to Pauling the atomic orbitals having slightly different energy and shape are intermixed to form hybrid orbital. These hybrid orbitals are involved in bond formation and these hybrid orbitals are equivalent in energy.
sp hybridisation-In this hybridisation one s and one p orbitals intermixes. Due to this intermixing two equivalent sp hybrid orbitals are formed. Similarly, in sp2 hybridisation one s and two p orbitals intermix. Due to this intermixing three equivalent sp2 hybrid orbitals are formed .Similarly in case of sp3 hybridisation one s and one p orbitals intermixes. Due to this intermixing four equivalent sp3 hybrid orbitals are formed.
Acetophenone has the chemical formula C6H5C(O)CH3.
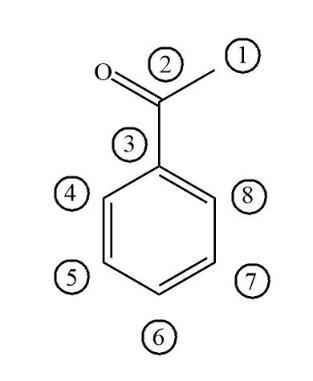
Above is the figure of Acetophenone. It has eight carbon atoms, in which six carbon atoms involved in the formation of benzene. Then one carbon is involved in the ketone functional group and the other carbon atom is involved in methyl.
First take a look at all benzene involved carbons i.e. the carbon numbers 3,4,5,6,7,8 .All these six carbons have 3 sigma bonds and 1 pi bonds connected to carbon atoms. So, the hybridisation of all six carbons which is involved in benzene is sp2 hybridised. Now take a look at carbonyl carbon i.e. carbon number 2 which has 3 sigma bonds and 1 pi bonds connected to oxygen.
Now take a look at carbon number 1,which is connected to one carbonyl carbon and three hydrogen atoms. So, the hybridisation of carbon 1 becomes sp3 hybridised.
Hence only one carbon i.e. carbon 1 becomes sp3 hybridised.
Note:
Thus in case of sp hybridisation one s and one p orbital involved so sp hybridisation has 50%s and 50\% $$$p$$ character. Similarly in s{p^2}hybridisationonesand two $$p$$ orbitals are involved sos{p^2}hybridisationhas33.33% sand66.66% p$$ character. While $s{p^3}$ in hybridisation one $s$ and three $$p$$ orbitals are involved so $s{p^3}$ hybridisation has $25\% $ $s$ and $75\% $ $75\% p$$ character.
