Question
Question: How many rings or pi bonds are present in a hydrocarbon with the formula \[{C_6}{H_{10}}\]? A) \(0...
How many rings or pi bonds are present in a hydrocarbon with the formula C6H10?
A) 0
B) 1
C) 2
D) 3
Solution
We must have to know that hydrocarbons are the compounds containing carbon and hydrogen atoms. Hydrocarbons are of two types: saturated and unsaturated. Saturated hydrocarbons contain single bonds between carbon atoms whereas unsaturated hydrocarbons contain double or triple bonds between carbon atoms.
Complete answer:
We must have to remember that the structure of C6H10 can be shown like this it’s a cyclohexane having one double bond in it and the rest are single bonds for carbons and hydrogen atoms. C6H10 is a benzene structure it has three double bonds or pi bonds having six carbon atoms and six hydrogen atoms. But in the given question it asked for C6H10 thus benzene is not possible since there are six carbon atoms it can be benzene but we also have to look for hydrogen atoms thus it is not fulfilling benzene structure. This hydrocarbon has given 6 carbon atoms and 10 hydrogen atoms thus this is the only way of fixing this compound like this.
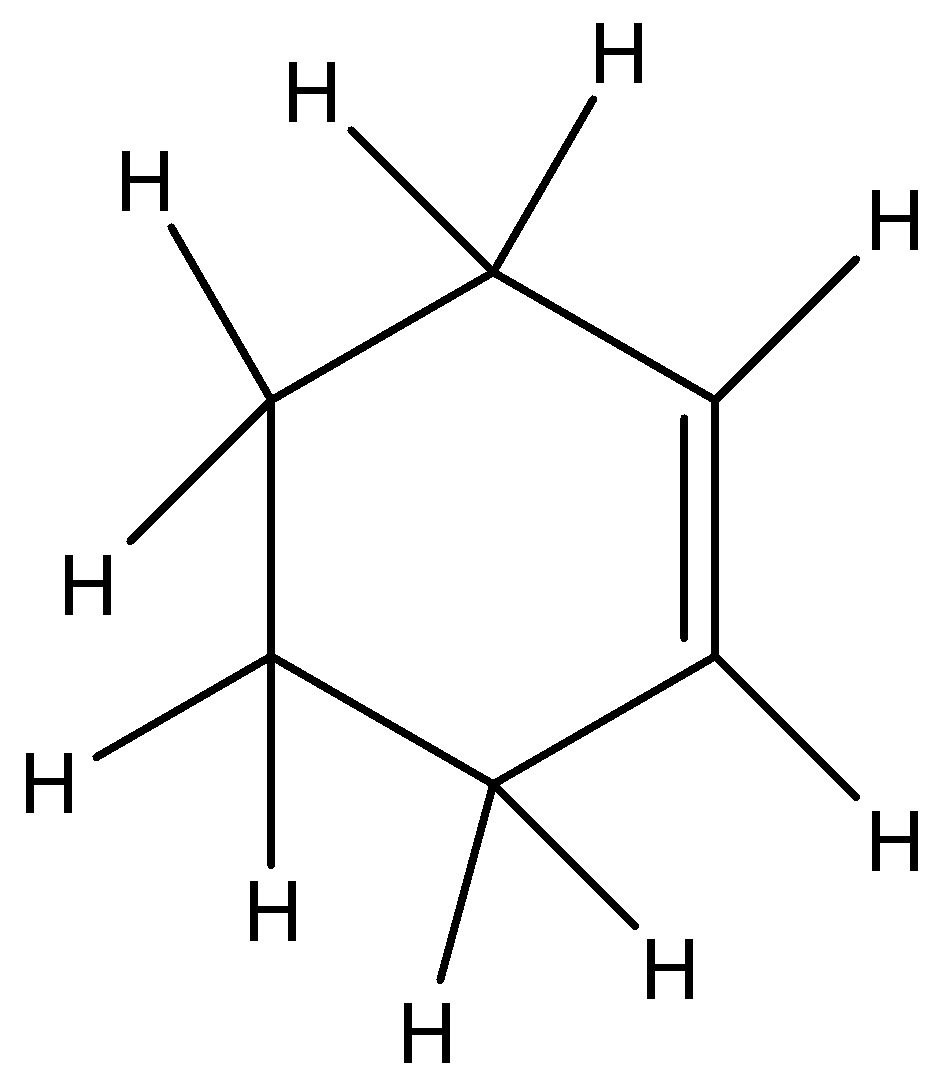
Option A) this is an incorrect option as this compound has one pi bond.
Option B) this is a correct option as C6H10 has one pi bond when we look at the structure of the hydrocarbon.
Option C) this is an incorrect option.
Option D) this is an incorrect option.
Note:
We also know that benzene is an unsaturated hydrocarbon whereas cyclohexane is a saturated hydrocarbon that contains six carbon atoms but the number of hydrogen atoms and bonds that is sigma and pi bond differs in number.
