Question
Question: How many right angles are there in a \[Xe{F_5}^ + \] ion?...
How many right angles are there in a XeF5+ ion?
Solution
Bases on the valence electrons of all atoms in a molecule, the molecular geometry and electron geometry will be expected. XeF5+ has octahedral as electron geometry and square pyramidal as molecular geometry. Based on the molecular geometry, right angles can be calculated.
Complete answer:
XeF5+ is a molecule consisting of Xenon as a central metal atom, and five fluorine atoms. The valence electrons on Xenon are 8 , and five fluorine atoms have 5×7=35 electrons, as each fluorine atom has 7 valence electrons. Thus, the total valence electrons on the given molecule will be 8+35−1=42 electrons.
The eight electrons on a xenon atom are involved in bond formation with five fluorine atoms and one electron was lost as it is a cation, the remaining two electrons will exist as a lone pair of electrons. Thus, the hybridization is sp3d2 . Thus, the electron geometry is octahedral, and the molecular geometry is square pyramidal due to the presence of one lone pair of electrons.
The structure of XeF5+ is
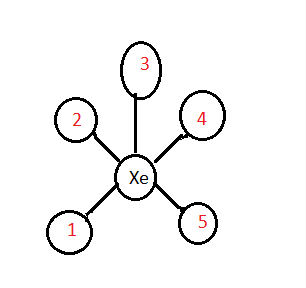
The five fluorine atoms were considered as 1,2,3,4, and 5 . Based on the above structure the right angles were:
3−Xe−1
3−Xe−2
3−Xe−4
3−Xe−5
1−Xe−2
1−Xe−5
4−Xe−5
2−Xe−4
Thus, there is a total of eight right angles in XeF5+
Note:
Right angle means the angle must be equal to 900 . In the above structure, there were eight right angles, meaning there were eight bonds in which the bond angle equal to 900 . The right angles can be considered based on the molecular geometry but not electron geometry.
