Question
Question: How many resonance structures are there for \({\text{N}}{{\text{O}}_{\text{2}}}\)?...
How many resonance structures are there for NO2?
Solution
For calculating the resonance structure of any species, first of all we should know about the number of electrons present in the valence shell and formal charge of each atom present in the species.
Complete Solution :
- Resonance structures are the type of hybrid structures of a single molecule, where different arrangements of electrons can be seen on the atoms present in that molecule.
Resonance structures of Nitrogen dioxide (NO2) will be predicted as follow:
-First we calculate the number of valence electrons in nitrogen and oxygen atoms. As we know that atomic number of nitrogen is 7 & its electronic configuration is 1s2,2s22p3, so from this it is clear that 5 valence electrons are present in the nitrogen atom. Similarly we know that atomic number of oxygen is 8 & its electronic configuration is 1s2,2s22p4, so from this it is clear that 6 valence electrons are present in the oxygen atom.
-Now we form Lewis dot structure for nitrogen dioxide molecule and that is shown as follow:
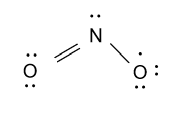
-Now we calculate the formal charge on nitrogen and oxygen atoms and formula to calculate formal charge is as follow:
Formal charge = no. of valence electrons – no. of covalent bond – no. of electrons present in lone pairs
Formal charge of nitrogen atom = 5−3−2=0
i.e. no charge is present on nitrogen atoms.
Formal charge of single bonded oxygen atom = 6−1−4=1
i.e. one negative charge is present on a single bonded oxygen atom.
Formal charge of double bonded oxygen atom = 6−2−4=0
i.e. no charge is present on a double bonded oxygen atom.
-So, resonance structures of NO2 is shown as follow:
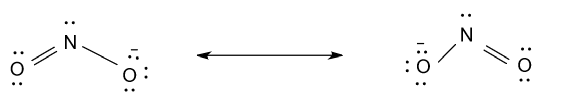
Hence, there are two resonance structures for NO2.
Note: Here some of you may think that why we show six valence electrons in place of five for a single bonded oxygen atom, so the reason is that the negative charge of that atom represents one extra electron from the original number of electrons.
