Question
Question: How many reactions give **CORRECT** products ? (i) 2Na + NH3 (liq) → Na₂NH + H₂↑ (ii) Na + (x+y) NH3...
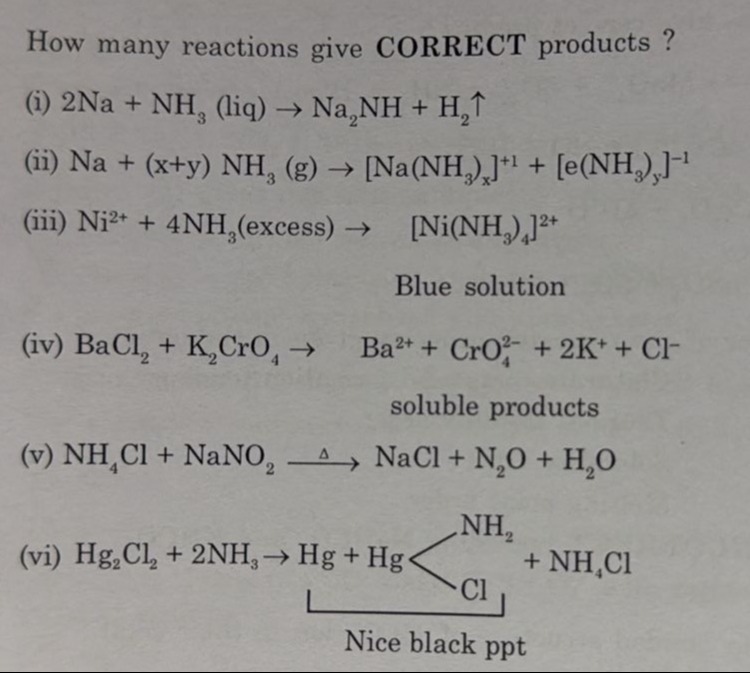
How many reactions give CORRECT products ? (i) 2Na + NH3 (liq) → Na₂NH + H₂↑ (ii) Na + (x+y) NH3 (g) → [Na(NH3)ₓ]⁺¹ + [e(NH3)ᵧ]⁻¹ (iii) Ni²⁺ + 4NH₃(excess) → [Ni(NH₃)₄]²⁺ Blue solution (iv) BaCl₂ + K₂CrO₄ → Ba²⁺ + CrO₄²⁻ + 2K⁺ + Cl⁻ soluble products (v) NH₄Cl + NaNO₂ △ NaCl + N₂O + H₂O (vi) Hg₂Cl₂ + 2NH₃ → Hg + Hg + NH₄Cl Nice black ppt
2
Solution
The correctness of each reaction is analyzed below:
(i) 2Na+NH3(liq)→Na2NH+H2↑. The reaction of sodium with liquid ammonia primarily yields sodium amide, NaNH2. The reaction shown is for the formation of sodium imide, which typically occurs at higher temperatures. Therefore, under the condition of liquid ammonia, this reaction is incorrect. The correct reaction is 2Na+2NH3(liq)→2NaNH2+H2↑.
(ii) Na+(x+y)NH3(g)→[Na(NH3)x]+1+[e(NH3)y]−1. This equation represents the dissolution of sodium in liquid ammonia, forming solvated sodium ions and solvated electrons, which is responsible for the characteristic blue color. The state of ammonia is given as (g), which is likely a typo and should be (liq). Assuming it is liquid ammonia, the products are correctly represented as solvated ions and electrons. This is a correct representation of the dissolution process.
(iii) Ni2++4NH3(excess)→[Ni(NH3)4]2+ Blue solution. Nickel(II) ion reacts with excess ammonia to form the hexaammine complex, [Ni(NH3)6]2+, which is violet-blue. While a tetraammine complex [Ni(NH3)4]2+ exists, the coordination number is usually 6 with excess ammonia in solution. The color is described as blue. However, the formula with excess ammonia is typically [Ni(NH3)6]2+. Thus, the product formula is likely incorrect with excess ammonia.
(iv) BaCl2+K2CrO4→Ba2++CrO42−+2K++Cl− soluble products. This is a reaction between barium chloride and potassium chromate. Barium chromate (BaCrO4) is insoluble and precipitates out. The reaction is BaCl2(aq)+K2CrO4(aq)→BaCrO4(s)+2KCl(aq). The products are solid BaCrO4 and soluble KCl. The statement that the products are soluble ions is incorrect.
(v) NH4Cl+NaNO2△NaCl+N2O+H2O. Heating a mixture of ammonium chloride and sodium nitrite produces nitrogen gas (N2), not nitrous oxide (N2O). The reaction is NH4Cl+NaNO2△NaCl+N2+2H2O. Therefore, this reaction is incorrect.
(vi) Hg2Cl2+2NH3→Hg+Hg(NH2)Cl+NH4Cl Nice black ppt. Mercurous chloride (Hg2Cl2) reacts with ammonia to form a mixture of black metallic mercury (Hg) and white mercuric amidochloride (Hg(NH2)Cl), which appears black due to the presence of finely divided mercury. Ammonium chloride is also formed. This reaction is correct.
Based on the analysis, reactions (ii) and (vi) are correct.
Final count of correct reactions is 2.
