Question
Question: How many products are formed in the given reaction? 
Solution
A carbene is an intermediate containing a neutral carbon atom with two unshared valence electrons. It is generally represented as R′−(:C)−R. If the two alkyl groups are replaced by hydrogen atoms, then the carbene is specifically known as methylene. Due to instability, the carbenes are very short lived and hence are very reactive in nature.
Complete answer:
For the given conditions, reaction mechanism is as follows:
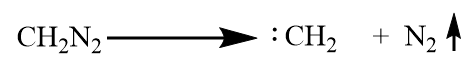
Step-1: Formation of carbene (methylene) from diazomethane:
Step-2: Reaction of carbene with the given compound.
Methylene reacts with the given compound and breaks the double bond of the ring and forms a three membered ring-like structure on the ring. Generally, in this reaction the stereochemistry of the double bond remains unchanged. The reaction proceeds as follows:
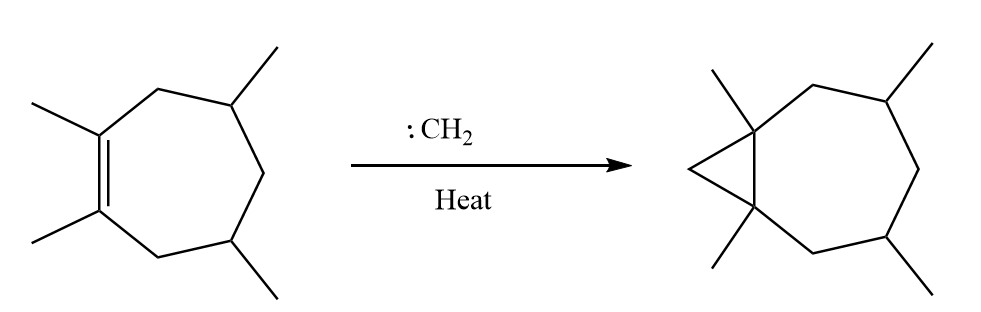
Now, the two possibilities with which the carbene can attack the double bond are as follows:
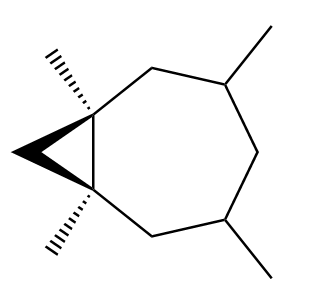
Possibility-1: Attack of methylene from above the plane of the double bond. The product formed in this case will be as follows:
Possibility-2: Attack of methylene from below the plane of the double bond. The product formed in this case will be as follows:
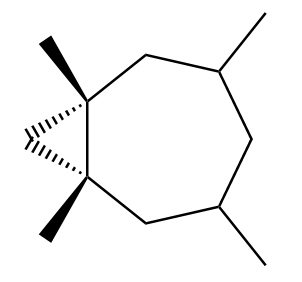
Although these compounds seem to be enantiomers, but if we rotate the product considered in possibility 1 to an angle of 180o, we observe that the new product formed will be the same as the product considered in possibility 2. So, we can conclude that both the products are identical to each other.
Hence, there is formation of only one product for the given reaction conditions.
Note:
It is important to note that the major difference between identical compounds and enantiomers is that in identical compounds, all the stereo centres have the same absolute configuration whereas in enantiomers, at least one chiral carbon will have a relatively opposite configuration.
