Question
Question: How many primary amines are possible with the molecular formula \({{\text{C}}_{4}}{{\text{H}}_{11}}\...
How many primary amines are possible with the molecular formula C4H11N?
A. 2
B. 3
C. 4
D. 5
Solution
Primary amines are 1o amines. Amines have the substituent −NH2 group. Primary amines (R−NH2) basically arise when one of hydrogen from ammonia molecule is replaced by alkyl group or phenyl group. The aliphatic primary amine includes methyl amine and aromatic primary amine includes aniline. The structures of primary amines are like,
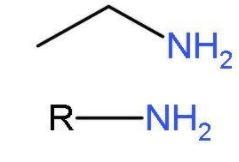
Structures of primary amines will be drawn by making isomers of by this molecular formula C4H11N.
Complete step by step answer:
Let us draw the primary amines with the molecular formula C4H11N, using the chain isomerism.
- Chain isomerism occurs when compounds with the same molecular formula have different arrangements in which the parent carbon chain is reduced by adding branches to the main chain.
- The alkane chain of the molecule C4H11N is butane as it has four carbon atoms.
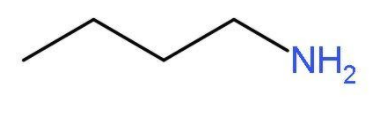
- The simplest primary amine will be butan-1-amine or butylamine. In this primary amine, the amino group is present at the first carbon atom of the butane chain. The molecular formula of butylamine will be CH3CH2CH2CH2NH2. The structure of butylamine is
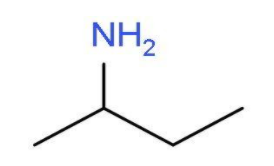
- The other primary amine which can be formed by shifting the position of the amine group will be butan-2-amine. In this primary amine, the amino group is present at the second carbon atom of the butane chain. The molecular formula of butan-2-amine will be CH3CHNH2CH2CH3. The structure of butan-2-amine is
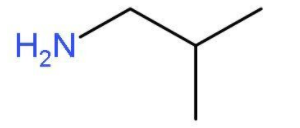
- The other primary amine which can be formed by chain isomerism will be 2-methylpropan-1-amine. In this primary amine, the amino group is present at the first carbon atom of the propane chain and one carbon atom is used as methyl group at second position. The molecular formula of 2-methylpropan-1-amine will be H2NCH2CH(CH3)2. The structure of 2-methylpropan-1-amine is
- The other primary amine which can be formed will be 2-methylpropan-2-amine. . In this primary amine, the amino group is present at the second carbon atom of the propane chain and one carbon atom is used as methyl group at second position. The molecular formula of 2-methylpropan-2-amine will be (CH3)3C−NH2. The structure of 2-methylpropan-2-amine is
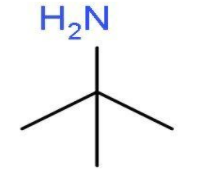
4 primary amines are possible with the molecular formula C4H11N, which is option ‘c’.
Note: 1o amines have no relation with the type of carbon it is attached. It means that 1o amine can be attached to primary carbon, secondary carbon and tertiary carbon atoms. 1o amines does not mean it will be attached to 1o carbon only. Please do not relate the degree of amines like this. It is the wrong definition and hence, you will get the wrong answer.
