Question
Question: How many position isomers of dibromo naphthalene are possible if each ring naphthalene has one halog...
How many position isomers of dibromo naphthalene are possible if each ring naphthalene has one halogen ?
Solution
The position isomer can be defined as the isomer which differs in position of the substituted atom in the chain or ring. The number of position isomers possible for naphthalene is equal to the number of hydrogen atoms present on the ring or we can say the number of replaceable hydrogen atoms present.
Complete step by step answer:
First, let us understand the structure of the compound given to us. We have dibromo i.e. two bromine atoms are there and a naphthalene ring is present. Further, it is said that each ring has one halogen i.e. one bromine atom.
We already know that naphthalene consists of two benzene rings that are fused. These rings are resonance stabilised and have many properties similar to benzene.
Each ring in naphthalene consists of one bromine atom substituted at any place.
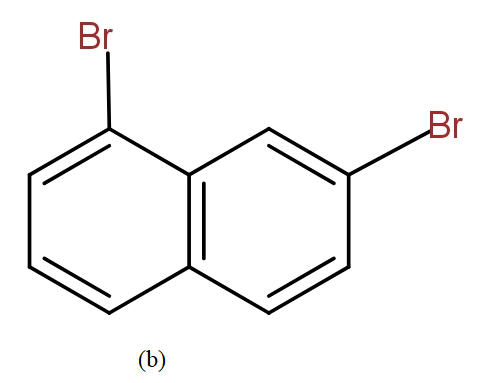
The position isomers of dibromo naphthalene can be drawn as follows-
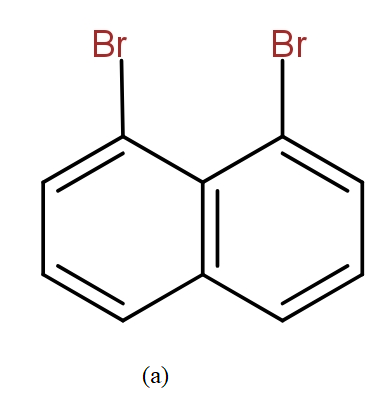
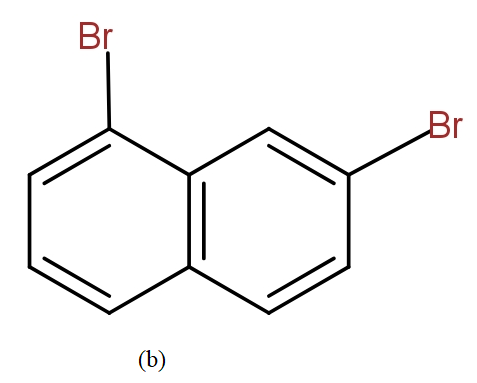
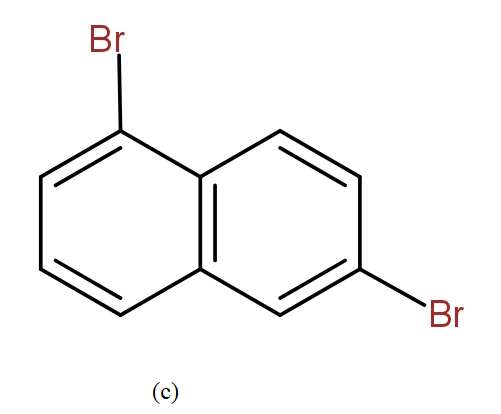
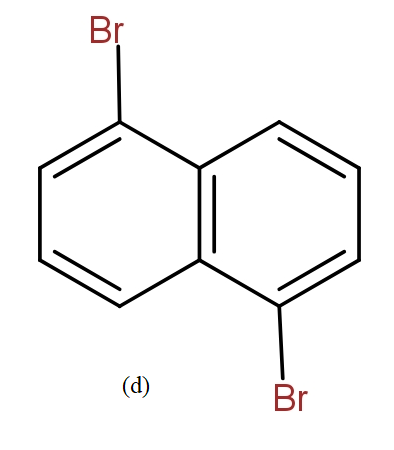
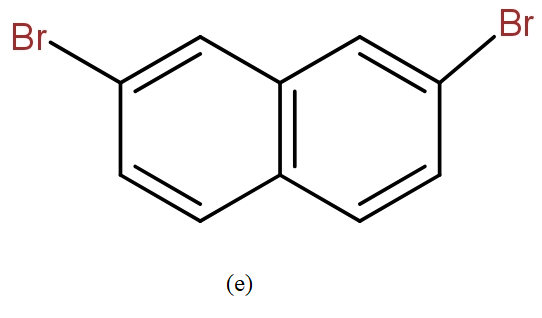
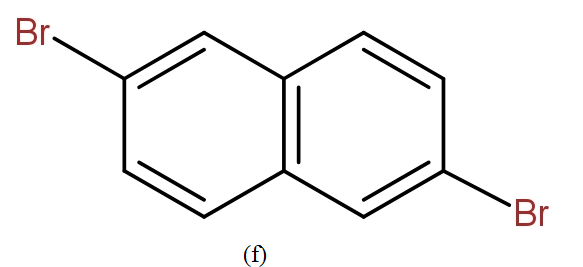
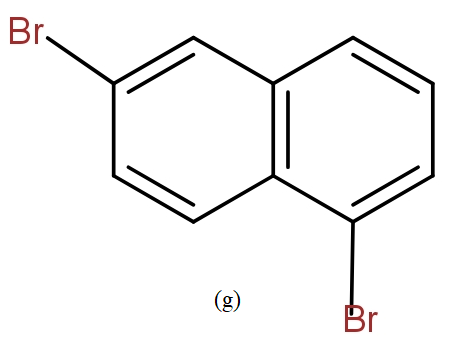
Thus, we can say that no more structure could be drawn.
So, the total number of position isomers of dibromo naphthalene are possible if each ring naphthalene has one bromine atom are 7.
Note: It must be noted that we can not say that the following structures could also be drawn and these are position isomers for dibromo naphthalene.
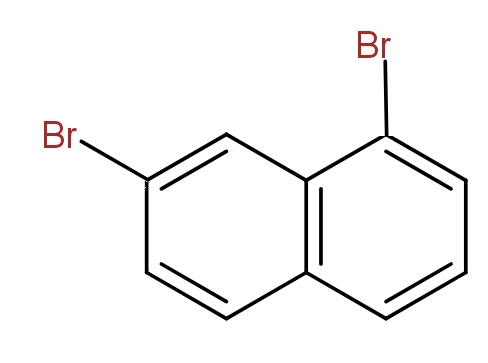
This is because this is the same as the structure (b) if we start numbering from the other side.
Thus, even other structures that could be drawn yield the same IUPAC name. So, they are not counted as different position isomers.
