Question
Question: How many pi bonds can an atom form?...
How many pi bonds can an atom form?
Solution
Pi bond is formed when the atomic orbitals overlap in such a manner that their axis remains parallel to each other and also their axis is perpendicular to the internuclear axis. The electrons involved in the formation of pi-bonds are called pi electrons.
Complete step-by-step answer: We should remember the fact that a pi bond can only be formed in the molecules which contains double and triple bonds (alkenes and alkynes).
Pi bonds cannot be formed in the compound containing a single bond. This is because whenever a pi bond is formed the compound shows restricted rotation and we know that a single bonded atom rotates freely and does not show restricted rotation.
We should remember that this type of sidewise overlapping always takes place after one of the three p-orbitals has already involved in axial overlapping forming a sigma bond. The rest of two p orbitals are symmetrically placed at right angles to each other and also to the overlapped p orbital. Therefore, these two orbitals are involved in the sidewise overlapping.
Hence, we can say that a pi bond always accompanies a sigma bond or is only formed after one sigma bond is formed in the compound.
Two pi bonds are maximum that can exist between a given pair of atoms.
Compound with double bond have one sigma and one pi bond as seen in the case of ethene as shown below:
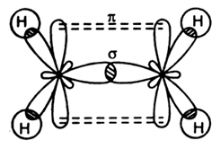
Compounds with triple bond have one sigma and maximum of two pi bonds as seen in the case of ethyne shown below:
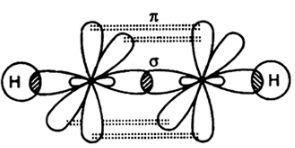
Compounds with quadruple bonds are very rare and can only be formed by the transition metals. And they consist of one sigma and two pi bonds and one delta bond.
Note: A pi bond formation shortens the bond distance between the two atoms involved. For example, C-C (sigma bond), C=C double bond (one sigma and one pi) have bond lengths as 154pm and 134pm respectively.
