Question
Question: How many pi bonds are present in caffeine?...
How many pi bonds are present in caffeine?
Solution
A single bond is formed of one sigma covalent bond, a double bond is formed of one sigma and one pi covalent bond and a triple bond is formed of one sigma and two pi covalent bonds.
Complete answer:
As we all are familiar with covalent bonds which are formed by the mutual sharing of electrons between the atoms and form a link between the atoms. There are mainly two types of covalent bond formed. First is the sigma covalent bond and the second is the pi covalent bond. The sigma covalent bond is formed by the end to end overlap of the two orbitals. The two orbitals can be two s-orbitals, two p-orbitals or one s-orbital or one p-orbital. The pi covalent bond is formed by side by side overlap of two parallel orbitals.
For finding the number of pi bonds, one must know that pi bond is present only in double bond and triple bond. In double bonds one sigma bond and one pi bond is present. In triple bonds one sigma bond and two pi bonds is present.
The caffeine is an alkaloid and the chemical formula of caffeine is C8H10N4O2.
The structure of caffeine is shown below.
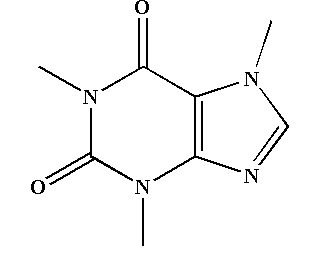
In the structure of caffeine, we can see that four double bonds are present, so the total number of pi bonds present in caffeine will be 4.
Note:
The structure of caffeine is formed by the fusion of pyrimidinedione ring and imidazole ring. The pyrimidinedione is a 6 membered ring which contains 2 nitrogen atoms and an imidazole ring is a 5 membered ring which contains 2 nitrogen atoms.
