Question
Question: How many pi bonds are in \(S{{O}_{2}}\)?...
How many pi bonds are in SO2?
Solution
Approach this question by making an expanded structure of the given compound so that we can clearly see each and every bond by which atoms are connected together. If there is only one bond between 2 atoms then it is a sigma bond and if there is more than 1 bond between 2 atoms even then there is one sigma bond and rest are pi bonds.
Complete answer:
Let us first discuss the chemistry of sigma and pi bonds:-
Sigma and pi bonds are among the types of covalent bonds that can be distinguished by the type of overlap between 2 atomic orbitals. Covalent bond is generally formed by overlapping of orbitals (or we may say by sharing electron pairs).
-Sigma Bond (σ): These are the strongest type of covalent bond because they are formed by head-to-head overlapping of atomic orbitals. The electrons which participate in sigma bonds are known as sigma electrons. These bonds can exist independently and play a role in determining the shape of the molecule.
-Pi Bond (π): These bonds are relatively weaker bonds and are formed by side-by-side overlapping of atomic orbitals. The electrons which participate in pi bond formation are known as pi electrons. They can’t exist independently without sigma bonds. Also these bonds play no role in determining the shape of molecules.
Generally, if (A) single bond: 1σ bond
(B) Double bond: 1 σ bond, 1π bond
(C) Triple bond: 1 σ bond, 2 π bonds and so on.
Now let’s draw the expanded structure of sulphur dioxide (SO2) so as to clearly see the number of bonds between each and every atom.
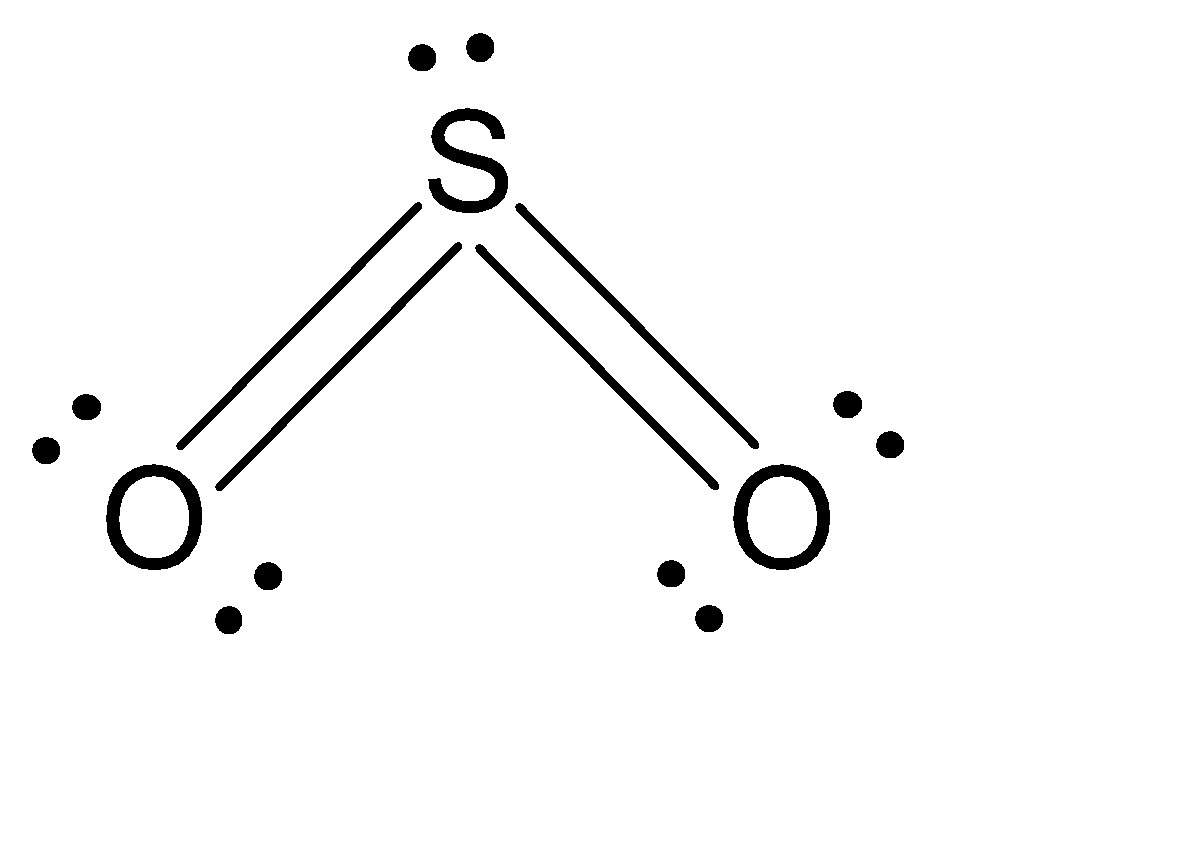
As we can see that, each atom is connected by at least one single bond which will be counted as σ bonds and the rest are π bonds.
So, the total number of sigma bonds are: 2 and the total number of pi bonds are: 2
Hence there are 2 pi (π) bonds in SO2.
Note:
The structure of SO2drawn above is the theoretically stable Lewis structure which means that theoretically this structure would be most stable than the hybrid structure of SO2 due to the fact that it has more covalent bonds and no formal charges on any of the atoms. But remember that experimental data would always point towards the hybrid structure.
