Question
Question: How many periods are there in long form of periodic table? A: \(6\) B: \(7\) C: \(8\) D: \(...
How many periods are there in long form of periodic table?
A: 6
B: 7
C: 8
D: 9
Solution
Periodic table is the tabular display of the chemical elements. In this table all the elements are arranged according to their atomic numbers. Periodic table gives information about many trends of the physical and chemical properties of the elements.
Complete step by step answer:
Long form of periodic table is also called the modern periodic table. In this table all the elements are arranged according to their increasing atomic numbers. This periodic table tells us about many physical and chemical trends of the elements. For example: as we go down the group size of the elements increases because the number of shells increases. Down the group all elements have similar atomic configuration. Along the period atomic numbers of the elements increase. Row of periodic tables is called period. There are a total of seven periods in the periodic table. Column of the periodic table is called group. There are a total of eighteen groups in the periodic table. So, there are a total of seven periods in the periodic tables.
So, the correct answer is Option B .
Additional Information:
Diagram of long form of periodic table is as follows:
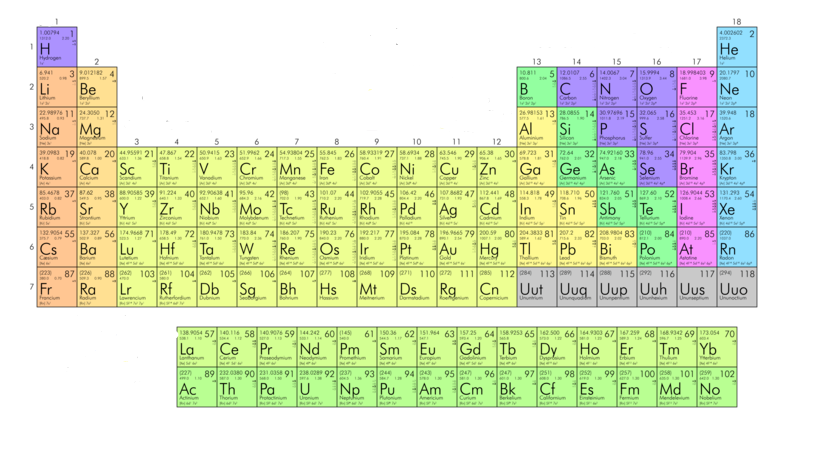
This picture shows the long form of periodic table. Elements characterized by different colored blocks have different meanings. Different color blocks tell that the elements with the same color block are of a particular category like metals, non-metals, alkali metals, alkaline earth metals, metalloids etc. below symbol of each element name of the element and its electronic configuration is written.
Note:
Down the group number of shells of the atom increases as a result size of the atom also increases. As the size increases, the hold of the nucleus on the outermost shell electron decreases and it becomes easy to remove the outermost shell electron.
