Question
Question: How many \(P-O-P\) linkage(s) is/are present in tetra polyphosphoric acid?...
How many P−O−P linkage(s) is/are present in tetra polyphosphoric acid?
Solution
There are four molecules of phosphoric acid in tetra polyphosphoric acid. There are a total of 3 oxygen linkages between the molecules in tetra polyphosphoric acid.
Complete step by step answer:
Tetra Polyphosphoric acid is a compound that is an oxoacid of phosphorus which belongs to group 15 of the p-block.
There are four molecules of phosphoric acid joined together to form tetra polyphosphoric whose formula is H6O13P4. The other names of tetra polyphosphoric acid are Hydroxy Phosphono phosphate and phosphono hydrogen phosphate.
The molecular weight or mass of Tetra Polyphosphoric is 337.94 g/mol.
The structure of Tetra Polyphosphoric is given below:
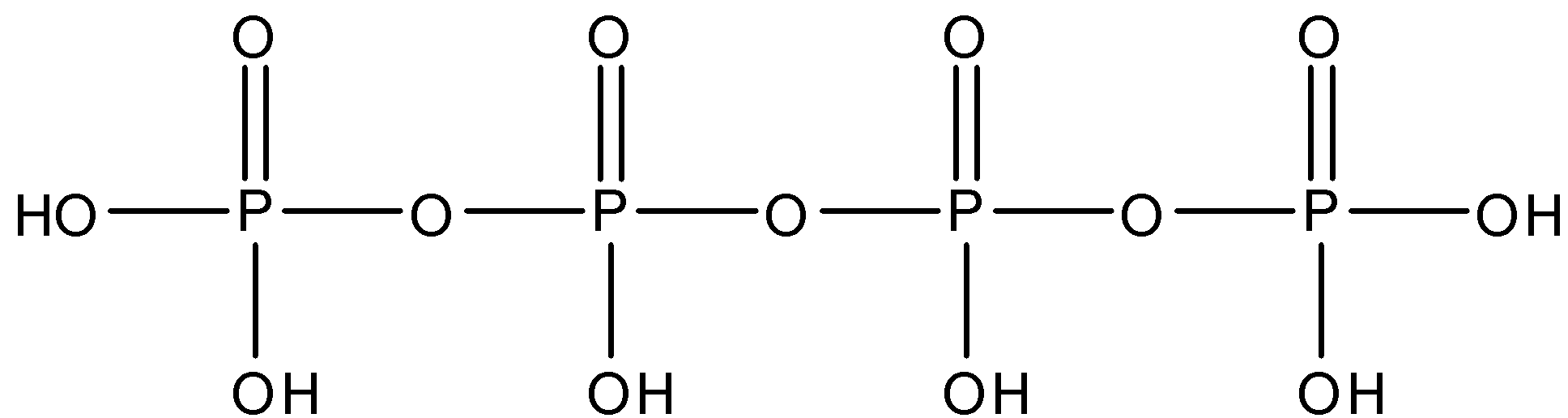
So, from the structure we can see that there are 3 oxygen linkages between the phosphorus molecules, each phosphorus is linked to an oxygen atom by a double bond. The terminal phosphorus molecules have two OH bonds each and both the middle phosphorus have one OH bond.
So, there are three P−O−P linkages present in tetra polyphosphoric acid.
Additional information:
Other oxoacids of phosphorus having P−O−P linkage(s) are given below:
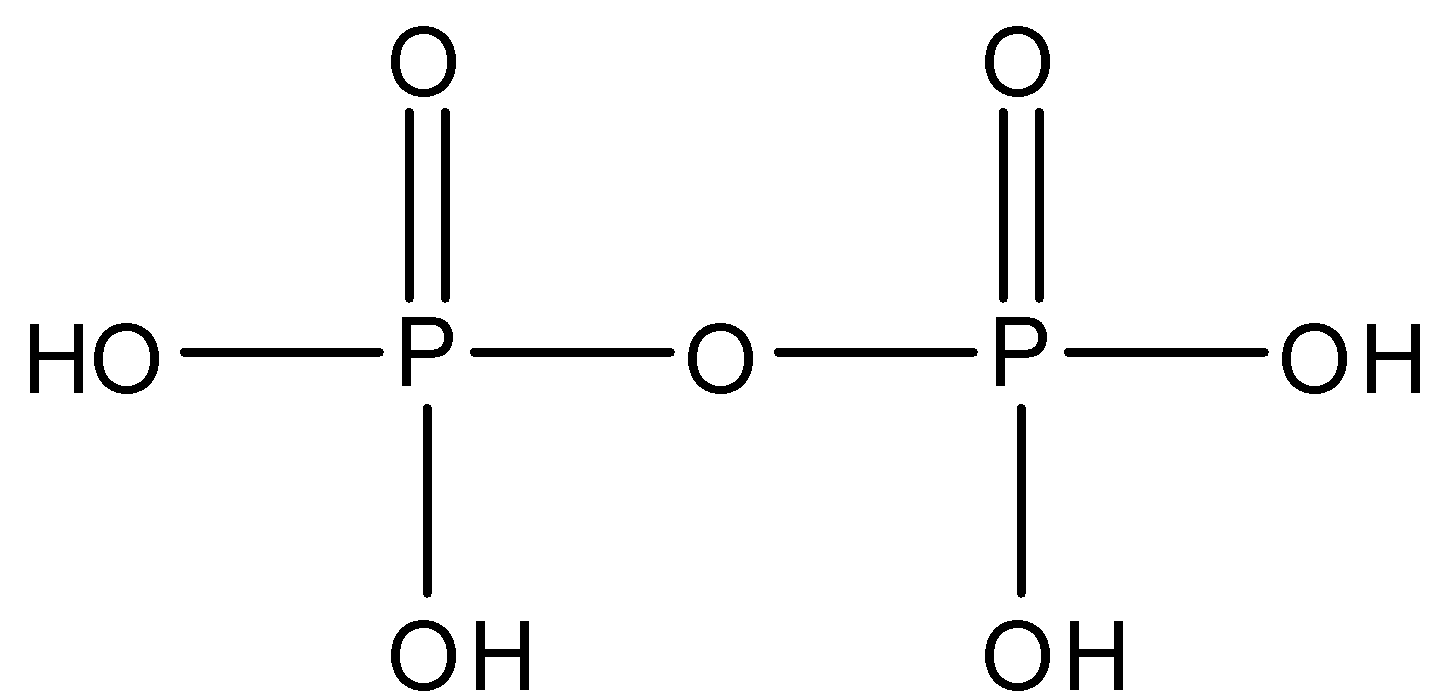
Pyrophosphoric acid whose formula is H4P2O7 and is made up of two molecules of phosphoric acid. There is one P−O−P linkage in pyrophosphoric acid. The structure is given below:
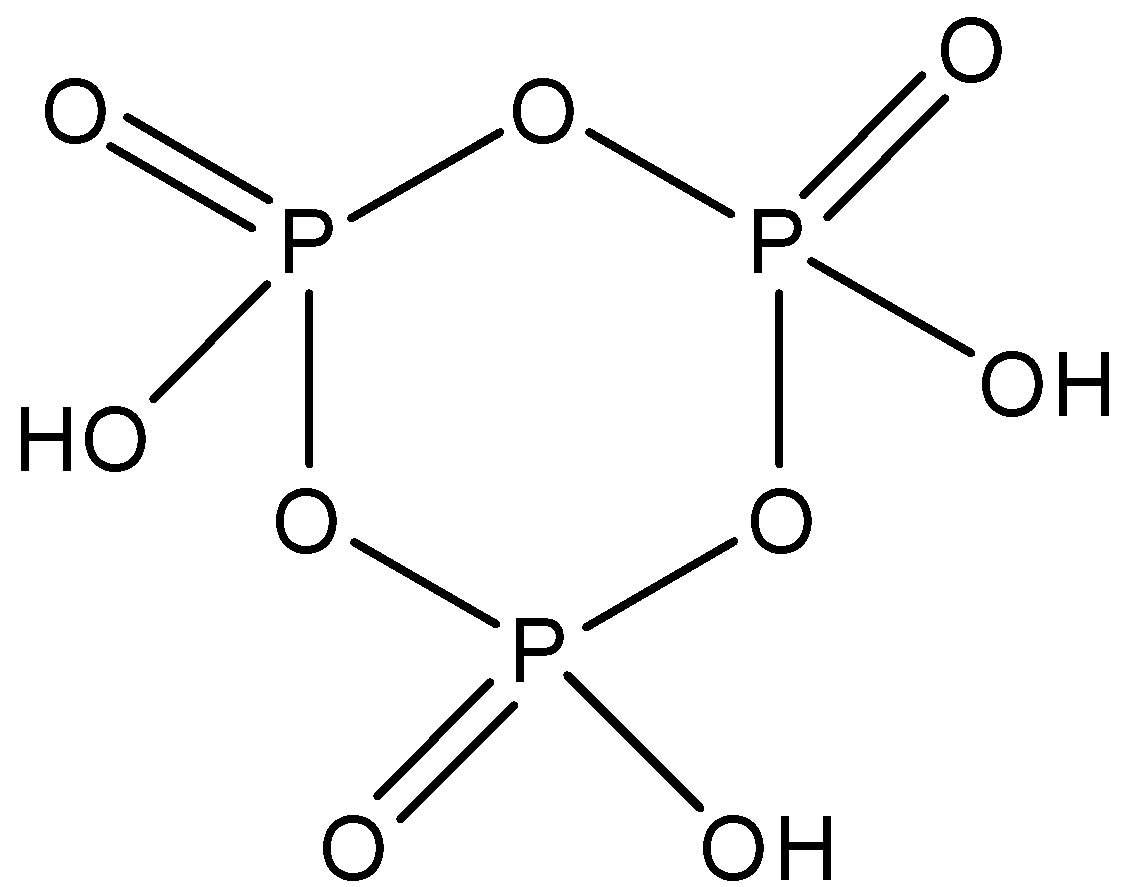
Cyclotrimetaphosphoric acid whose formula is (HOP3)3 and is made up of three molecules of phosphoric acid. It has a cyclic structure and there are three P−O−P linkages present between the phosphorus molecules. The structure is given below:
Note: The oxidation state of phosphorus in tetra polyphosphoric acid is +5. The oxidation state of phosphorus in Pyrophosphoric acid is +5 and in Cyclotrimetaphosphoric acid, the oxidation state of phosphorus is also +5. All these molecules are neutral.
