Question
Question: How many P-O-P bonds are present in cyclic meta-phosphoric acid? Also, give it structure....
How many P-O-P bonds are present in cyclic meta-phosphoric acid? Also, give it structure.
Solution
Molecular formula of cyclic meta-phosphoric acid is (H3O9P3). To find the number of P-O-P bonds in cyclic meta-phosphoric acid, we need to refer to its structure and then count the number of P-O-P bonds.
Complete step by step solution:
First, let us draw the structure of cyclic meta-phosphoric acid(H3O9P3).
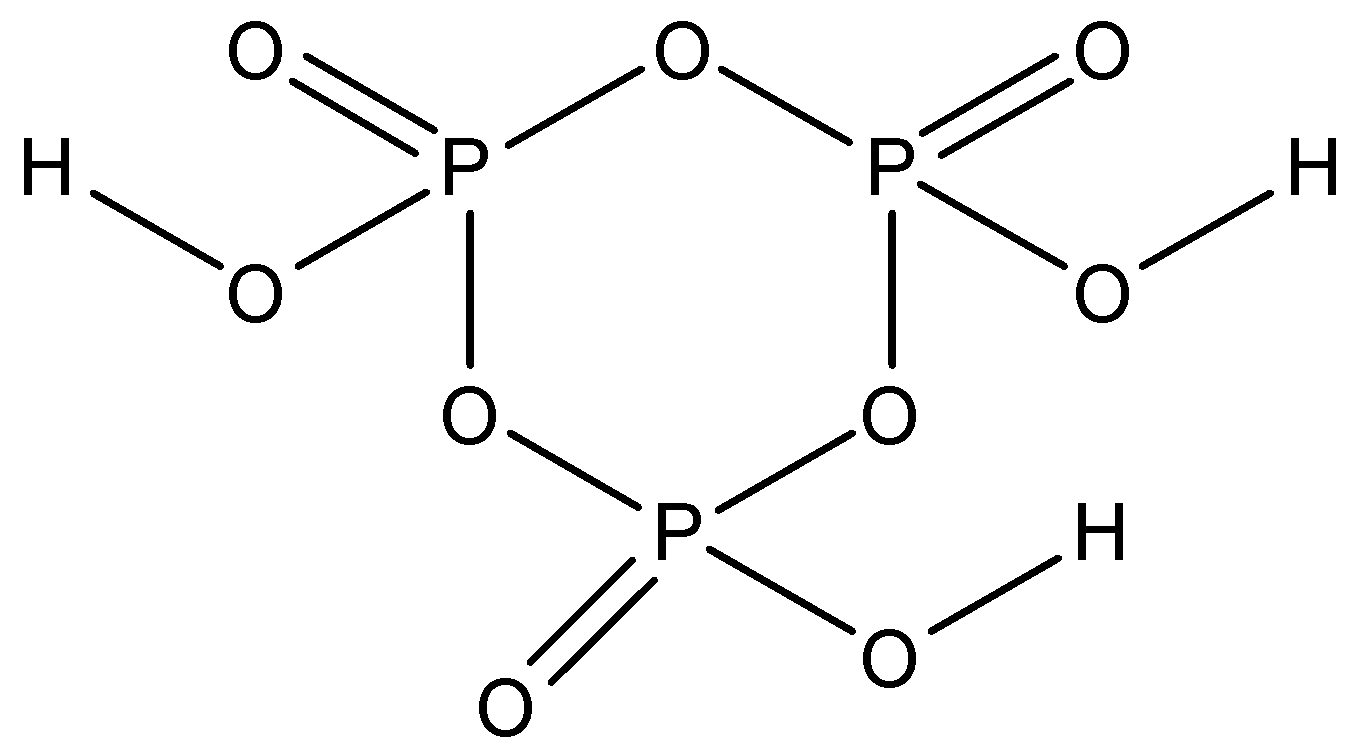
Now as we can see in the above structure, there are 3 types of bonds:P=O ,P−O−P and O−H bonds. We have been given to find the number of P-O-P bonds, so count the number of P-O-P bonds in the above drawn structure.
It is clear from the structure that there are three P-O-P bonds in the structure of cyclic meta-phosphoric acid. Hence, this is the required answer.
Additional information: Cyclic meta-phosphoric acid is (H3O9P3)is also known as cyclo triphosphate acid, trimetaphosphoric acid, and trimetaphosphate. It belongs to the class of inorganic compounds called non-metal phosphates. Cyclic meta-phosphoric acid is the cyclic anhydride of triphosphoric acid which is (H5P3O10) while ATP (Adenosine triphosphate) are the esters of triphosphoric acid.
Note: You can also be asked about the total number of sigma and pi-bonds in the cyclic meta-phosphoric acid. So, there are a total of fifteen bonds in the structure of cyclic meta-phosphoric acid and out of these fifteen bonds, there are twelve sigma bonds (σ) and three pi-bonds (π).
