Question
Question: How many P = O bonds and P – OH bonds (respectively) are present in orthophosphoric acid? A.2,1 ...
How many P = O bonds and P – OH bonds (respectively) are present in orthophosphoric acid?
A.2,1
B.3,3
C.1,3
D.4,3
Solution
Orthophosphoric acid’s chemical formula is H3PO4 Orthophosphoric acid, also known as Phosphate. It is a Triprotic acid means that the orthophosphoric acid molecule can dissociate up to three times, producing a hydrogen cation, H+, each time. It is a non-volatile syrupy liquid.
Complete step by step answer:
Orthophosphoric acid is a weak acid containing 4 atoms of Oxygen, 3 atoms of Hydrogen and 1 atom of Phosphorus. It can be conveniently prepared by dissolving P2O5 in water. Orthophosphoric acid also called as Phosphoric acid is a colorless soluble solid tribasic acid used in manufacture of fertilizers and soaps. The name "orthophosphoric acid" is actually used to differentiate this specific acid from other phosphoric acid, example Pyrophosphoric acid .Phosphoric acid often means this specific compound; and this name is used as the IUPAC Name. The chemical formula of phosphoric acid is H3PO4. Its molecular formula is written as H3O4P
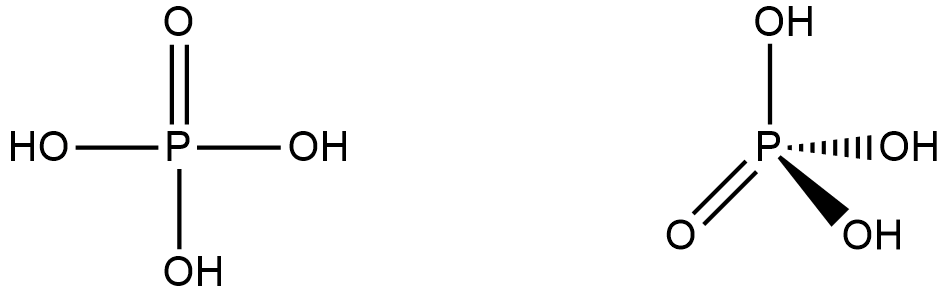
The central phosphorus atom is connected to an oxygen atom through a double bond and to three hydroxyl ( - OH) groups through single bonds. So there are 1 P = O bond, and 3 P - OH bond
Thus, Option C is correct.
Additional information:
Phosphoric acid was prepared first by Robert Boyle in 1694 by dissolving phosphorus pentoxide in water. Molecular Weight of H3PO4=97.995 g/mol
It is normally recognized as a colorless liquid with 85% concentration in water. The pure compound is a colorless solid. All three hydrogen are acidic in nature of different degrees and can leave molecules as H+ ions. When all three H+ ions are removed, orthophosphate ions are formed, commonly called "phosphate". When one or two hydrogen atoms are removed, dihydrogen phosphate ions are formed, and then finally hydrogen phosphate ions are formed.. Although phosphoric acid is not a strong acid but can still severely irritate the skin and damage the eyes.
Note:
Phosphoric acid is made from phosphorus, which is found naturally in the body and works with calcium to form strong bones and teeth. Phosphoric acid makes cola actually more acidic than lemon juice or vinegar. Phosphoric acid has been detected in multiple bio fluids, such as feces, urine, and blood. Orthophosphoric acid also forms esters, known as organophosphates .Phosphoric acid is generally seen in chemical laboratories as an 85% dilute solution, which is a colorless, odorless.
