Question
Question: How many \(P-H\) bonds are there in \({{H}_{3}}P{{O}_{2}}\)?...
How many P−H bonds are there in H3PO2?
Solution
H3PO2 is known by the name hypophosphorous acid and phosphonic acid. It is a type of oxyacid which can be defined as an acid which contains oxygen; this is phosphorus oxyacid. It is a powerful reducing agent.
Complete step-by-step answer: Hypophosphorous is colorless in nature have low melting point which is soluble in water, dioxane and alcohols. The molecular formula for the hypophosphorous acid is written as H3PO2 but the most appropriate way to write hypophosphorous acid is HOP(O)H2 HOP(O)H2 which suggests that the compound is monoprotic in nature where monoprotic can be defined as that characteristic of compound of being an acid which is capable of donating a proton and also capable of forming a covalent bond with an electron pair.
Hence to know about the bonds present in hypophosphorous acid we first draw the structure of the given compound which is as follows:
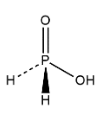
In the structure we can see that metal phosphorus is connected with four bonds in which two phosphorus bonds are connected with oxygen atoms and other two bonds are connected with hydrogen bonds through double, single, dashed and wedge bonds where all different bonds have different meanings.
Thus we can say that there only two P−H bonds are there in H3PO2.
Note: Hypophosphorous acid has many industrial and chemical applications. Its main industrial use is nickel plating through the process of electroplating and another main use is it act as an reducing agent which is generally used in the reaction where chromium(III) oxide reduces in chromium(II) oxide.
