Question
Question: How many oxygen atoms have \( - 1\) oxidation state in one mole product containing \(Cr\)? \({K_2}...
How many oxygen atoms have −1 oxidation state in one mole product containing Cr?
K2Cr2O7+H2SO4+H2O2low temperatureether
Solution
The number of electrons gained or lost by an element of a compound while formation of a chemical bond is known as its oxidation state. In a chemical reaction, if the oxidation state of an element increases, then it is said to be oxidized while if the oxidation state decreases, then it is said to be reduced.
Complete answer: As per question, the given reaction is as follows:
K2Cr2O7+H2SO4+H2O2low temperatureether
It is a redox reaction in which potassium dichromate reacts with hydrogen peroxide and sulphuric acid and gets reduced to chromium (III) sulphate along with the formation of potassium sulphate, water and dioxygen. The reaction proceeds as follows:
K2Cr2O7+4H2SO4+3H2O2low temperatureetherCr2(SO4)3+7H2O+K2SO4+3O2
The oxidation state of chromium in K2Cr2O7 is +6 whereas the oxidation state of chromium in Cr2(SO4)3 is +3. Hence potassium dichromate gets reduced during the reaction process and acts as an oxidizing agent.
Now, the structure of Cr2(SO4)3 is as follows:
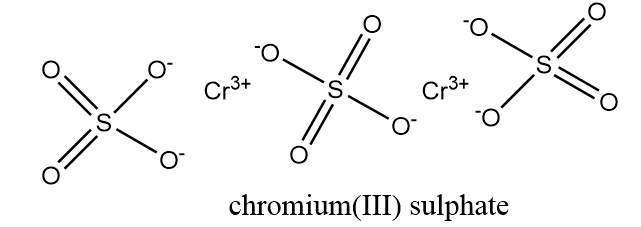
From the structure of Cr2(SO4)3, it is clear that all the oxygen atoms connected via single bond will be present in their −1 oxidation state and all oxygen atoms connected to sulphur via double bond will be present in their −2 oxidation state.
Hence, the number of oxygen atoms have −1 oxidation state in one mole product containing Cr=6.
Note:
It is important to note that trivalent chromium compounds are amphoteric in nature and chromium (III) sulphate generally exist in its hydrated form i.e., in the form of Cr2(SO4)3.xH2O where the value of x lies in the range of 0 to 18.
