Question
Question: How many optically active stereoisomers are possible for butane-2,3-diol? A.1 B.2 C.3 D.4...
How many optically active stereoisomers are possible for butane-2,3-diol?
A.1
B.2
C.3
D.4
Solution
The compounds having the same molecular as well as same structural formulae but differing in the relative arrangement of the atoms or groups in space are called stereoisomers and the phenomenon is termed as stereoisomerism. There are six geometrical isomers possible for dimethyl cyclohexane.
Complete step by step answer:
Let’s discuss optically active stereoisomers in detail. The stereoisomers that can rotate plane polarized light are called as optically active whereas an optically inactive compound is not capable of optical rotation.
Now, come to the question. Here, we have to identify the optically active stereoisomers possible for butane-2,3-diol.
There are three stereoisomers of butane-2,3-diol. They are as follows:
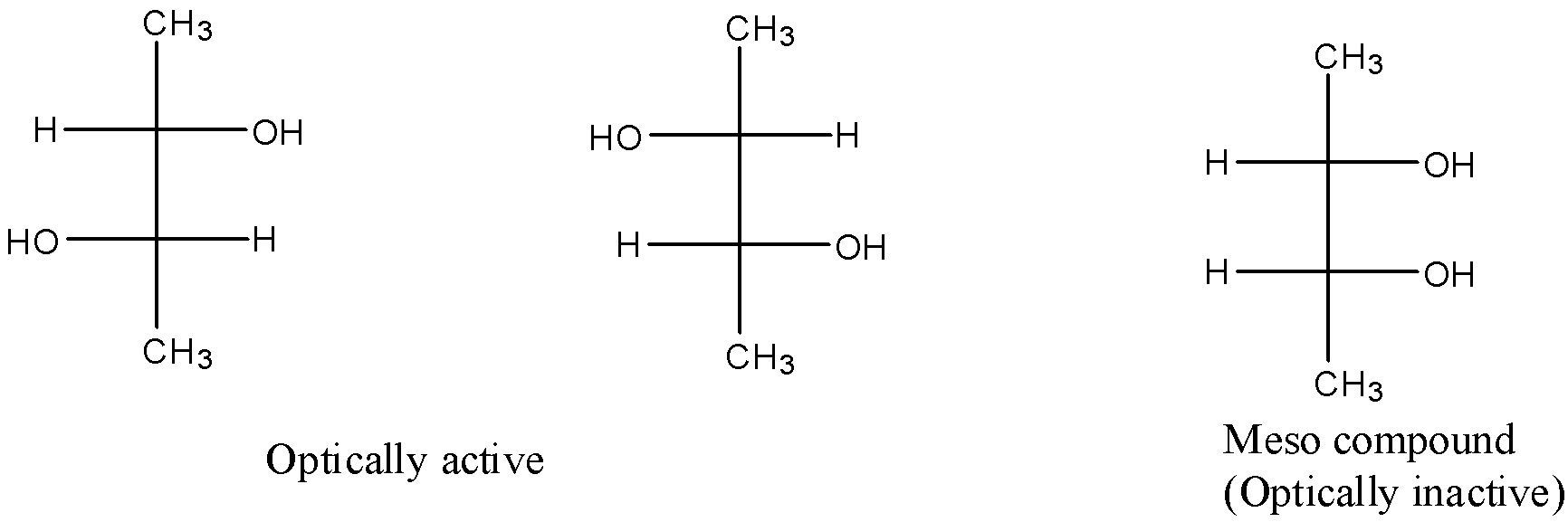
So, there are two optically active stereoisomers possible for butane-2,3-diol. The optically active stereoisomers are d, l isomers. The meso compound is optically inactive because of the internal compensation.
So, the correct answer is Option B.
Additional information:
Let’s discuss isomerism in detail. Always remember that isomerism is the phenomenon in which the compounds have the same chemical formula but their structure is different. There are two types of isomerism that is structural isomerism and stereoisomerism. Structural isomerism is shown by the compounds having the same molecular formulae differing in the arrangement of the atoms.
Note: There are two types of stereoisomers namely, enantiomer and diastereomer. Enantiomers are the two compounds that have nonsuperimposable mirror images of each other. Diastereomers are the two stereoisomers that are not mirror images of each other. Geometriacl isomers (cis and trans) are a type of diasteromer.
