Question
Question: How many of the following regents can distinguish between phenol and cyclohexanol? (1) Na (2) anh. $...
How many of the following regents can distinguish between phenol and cyclohexanol? (1) Na (2) anh. ZnCl2 + conc. HCl (3) NaNH2 (4) Na2Cr2O7 (5) Neutral FeCl3 (6) (NH4)2[Ce(NO3)6] (7) Br2/H2O
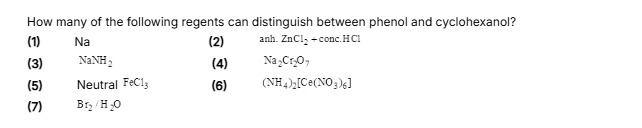
Answer
5
Explanation
Solution
- Reagent 2 (Lucas Reagent): Cyclohexanol (2° alcohol) reacts to form turbidity; phenol does not react.
- Reagent 4 (Na2Cr2O7/Acid): Cyclohexanol is oxidized (color change from orange to green); phenol is resistant to oxidation.
- Reagent 5 (Neutral FeCl3): Phenol gives a characteristic color; cyclohexanol does not.
- Reagent 6 (CAN): Phenol gives a color; cyclohexanol does not.
- Reagent 7 (Br2/H2O): Phenol forms a white precipitate and decolorizes Br2; cyclohexanol does not.
- Reagents 1 (Na) and 3 (NaNH2) react with both compounds, producing similar observable gas evolution (H2 and NH3 respectively), hence they are not distinguishing.
