Question
Question: How many of the following compounds have peroxide linkage? \[{{H}_{3}}P{{O}_{5}},\text{ }{{H}_{2}...
How many of the following compounds have peroxide linkage?
H3PO5, H2SO5, H2S2O8, HNO4, H4P2O7, H2S2O3, H2S2O6
A. 4
B. 5
C. 6
D. None of these
Solution
If there is a chemical bond between two oxygen atoms in a molecule then the chemical bond is called peroxide bond. The peroxide bonds are very less stable in the presence of sunlight and we have to keep them away from the sunlight.
Complete answer:
- In the question it is given how many of the given compounds contain peroxide bonds.
- We have to find the number of compounds that have peroxide bonds in their structures.
- The structure of H3PO5 is as follows.
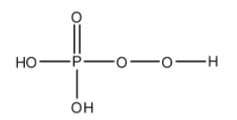
- H3PO5 has one peroxide bond in its structure.
- The structure of H2SO5 is as follows.
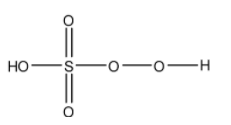
- H2SO5 has one peroxide bond in its structure.
- The structure of H2S2O8 is as follows.
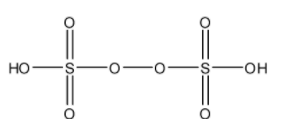
- H2S2O8 has one peroxide bond in its structure.
- The structure of HNO4 is as follows.
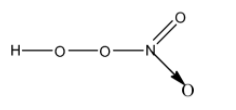
- HNO4 has one peroxide bond in its structure.
- The structure of H4P2O7 is as follows.
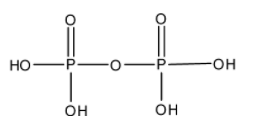
- There is no peroxide bond in H4P2O7 .
- The structure of H2S2O3 is as follows.
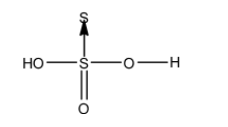
- There is no peroxide bond in H2S2O3
- The structure of H2S2O6 is as follows.
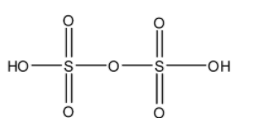
- There is no peroxide bond in H2S2O6
- Out of the given seven compounds only four compounds have peroxide bonds in their structure.
So, the correct option is A.
Note:
Without drawing or knowing the structure of the compounds we cannot say whether the compound contains a peroxide bond or not. The structure of the molecules or compounds reveals the presence of the peroxide bond.
