Question
Question: How many of the following complexes are paramagnetic?...
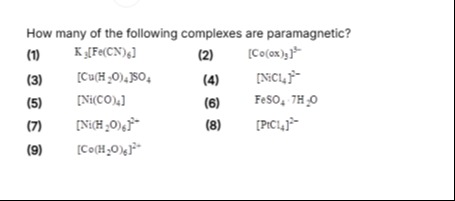
How many of the following complexes are paramagnetic?
A
K3[Fe(CN)6]
B
[Co(ox)3]3−
C
[Cu(H2O)4]SO4
D
[NiCl4]2−
E
[Ni(CO)4]
F
FeSO4.7H2O
G
[Ni(H2O)6]2−
H
[PtCl4]2−
I
[Co(H2O)6]2+
Answer
5
Explanation
Solution
To determine if a complex is paramagnetic, we need to find the number of unpaired electrons in the central metal ion.
- K3[Fe(CN)6]: Fe3+ (d5), strong field CN− → low spin t2g5 → 1 unpaired electron. Paramagnetic.
- [Co(ox)3]3−: Co3+ (d6), strong field ox2− → low spin t2g6 → 0 unpaired electrons. Diamagnetic.
- [Cu(H2O)4]SO4: Cu2+ (d9) → 1 unpaired electron. Paramagnetic.
- [NiCl4]2−: Ni2+ (d8), weak field Cl−, tetrahedral → 2 unpaired electrons. Paramagnetic.
- [Ni(CO)4]: Ni0 (3d84s2), strong field CO, tetrahedral → 0 unpaired electrons. Diamagnetic.
- FeSO4.7H2O: Fe2+ (d6), weak field H2O, octahedral → high spin t2g4eg2 → 4 unpaired electrons. Paramagnetic.
- [Ni(H2O)6]2−: Ni2− (3d104s2) → 3d10 is filled → 0 unpaired electrons. Diamagnetic.
- [PtCl4]2−: Pt2+ (d8), square planar → 0 unpaired electrons. Diamagnetic.
- [Co(H2O)6]2+: Co2+ (d7), weak field H2O, octahedral → high spin t2g5eg2 → 3 unpaired electrons. Paramagnetic.
The paramagnetic complexes are (1), (3), (4), (6), and (9). There are 5 paramagnetic complexes.
