Question
Chemistry Question on coordination compounds
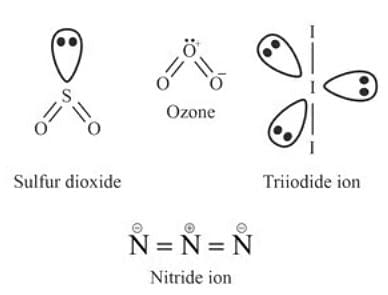
How many of the following are bent in shape?
SO2, O3, I3-, N3-
Answer
SO2 (Sulfur Dioxide) has a bent molecular geometry due to the presence of a lone pair of electrons on sulfur atom, which distorts the molecular shape.
O3 (Ozone) has central oxygen atom is surrounded by two unshared electrons (one lone pair) and six bonding electrons (three bonds). So it is bent in shape.
I3- (Triiodide ion), and N3- (Azide ion) are linear.
Therefore,
SO2 (Sulfur Dioxide) - Bent shape
O3 (Ozone) - Bent shape
I3- (Triiodide ion) - Linear shape
N3- (Azide ion) - Linear shape
So, Two molecules/ions, SO 2 and O3, have a bent shape. The other two, I3- and N3-, have a linear form.