Question
Question: How many of reagents can carry out following transformation: (a) Cu, 300°C (b) O₃ / Zn, H₂O (c) NBS...
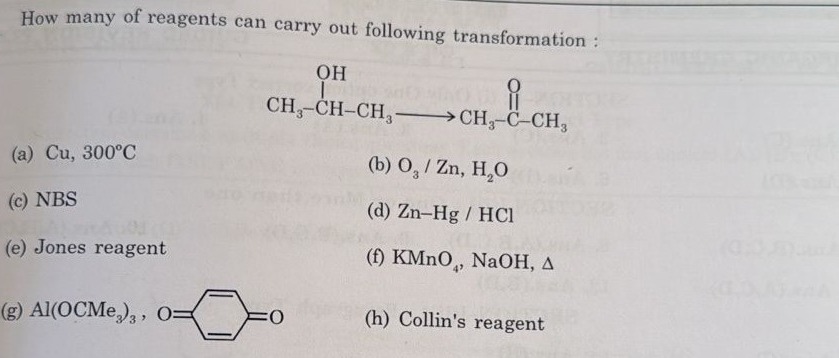
How many of reagents can carry out following transformation:
(a) Cu, 300°C (b) O₃ / Zn, H₂O (c) NBS (d) Zn-Hg / HCl (e) Jones reagent (f) KMnO₄, NaOH, Δ (g) Al(OCMe₃)₃, (h) Collin's reagent
1
2
3
4
5
5
Solution
The transformation is the oxidation of propan-2-ol to propanone:
CH3−CH(OH)−CH3→CH3−C(=O)−CH3
This is the oxidation of a secondary alcohol to a ketone.
Let's examine each reagent:
(a) Cu,300∘C: Hot copper is a catalyst for the dehydrogenation of alcohols. Secondary alcohols are dehydrogenated to ketones. So, propan-2-ol is oxidized to propanone. This reagent works.
(b) O3/Zn,H2O: Ozonolysis is used to cleave carbon-carbon double or triple bonds. It is not used for the oxidation of saturated alcohols. This reagent does not work.
(c) NBS: N-Bromosuccinimide is primarily used for allylic and benzylic bromination. While it can act as an oxidant under specific conditions (e.g., in the presence of DMSO), NBS alone is not a standard reagent for oxidizing simple secondary alcohols to ketones. This reagent does not work in this context.
(d) Zn-Hg/HCl: This is Clemmensen reduction, used to reduce carbonyl compounds to alkanes. This is the reverse reaction of what is desired. This reagent does not work.
(e) Jones reagent: Jones reagent (CrO3 in aqueous sulfuric acid with acetone) is a strong oxidizing agent that oxidizes secondary alcohols to ketones. This reagent works.
(f) KMnO4,NaOH,Δ: Alkaline potassium permanganate is a strong oxidizing agent that oxidizes secondary alcohols to ketones. This reagent works.
(g) Al(OCMe3)3, p-benzoquinone: This is a system for Oppenauer oxidation, which oxidizes secondary alcohols to ketones using an aluminum alkoxide catalyst and a hydrogen acceptor (in this case, p-benzoquinone). This reagent works.
(h) Collin's reagent: Collin's reagent (CrO3⋅2Py) is a mild oxidizing agent that oxidizes primary alcohols to aldehydes and secondary alcohols to ketones. This reagent works.
The reagents that can carry out the transformation are (a), (e), (f), (g), and (h). Counting these reagents, there are 5.
