Question
Question: How many number of product are possible for the following reaction? ...
How many number of product are possible for the following reaction?
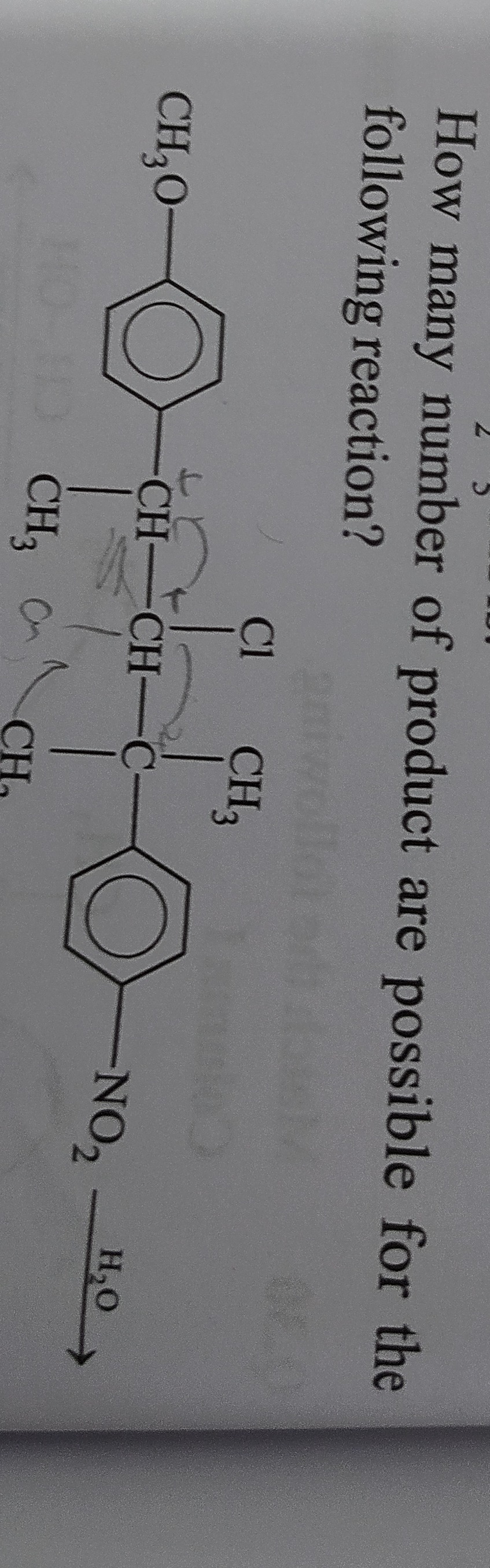
18
Solution
The reaction proceeds via an S_N1/E1 mechanism due to the secondary alkyl halide and weak nucleophile/solvent (H₂O). This involves the formation of carbocations and subsequent rearrangements to more stable forms. Both substitution (S_N1) and elimination (E1) products are possible from each carbocation.
-
Initial Carbocation (Secondary): Formed at the carbon bearing the chlorine. This carbocation can undergo direct substitution (forming an alcohol) or elimination (forming an alkene).
- Substitution product: Contains two chiral centers, leading to 4 stereoisomers (assuming racemic starting material or considering all possibilities).
- Elimination product: Forms an alkene which exhibits E/Z isomerism, leading to 2 stereoisomers.
-
Rearranged Carbocations (Tertiary):
- 1,2-Hydride shift: Leads to a highly stable tertiary benzylic carbocation.
- Substitution product: Contains one chiral center, leading to 2 stereoisomers.
- Elimination product: Forms the same alkene as from the initial carbocation, leading to 2 stereoisomers (E/Z).
- 1,2-Methyl shift: Leads to a less stable tertiary benzylic carbocation (compared to the hydride-shifted one).
- Substitution product: Contains two chiral centers, leading to 4 stereoisomers.
- Elimination products: Two different alkenes are possible.
- One alkene has a chiral center and E/Z isomerism, leading to 4 stereoisomers.
- Another alkene has a chiral center but no E/Z isomerism, leading to 2 stereoisomers.
- 1,2-Hydride shift: Leads to a highly stable tertiary benzylic carbocation.
Summing up all unique stereoisomeric products: 4 (from initial S_N1) + 2 (from hydride-shifted S_N1) + 4 (from methyl-shifted S_N1) + 2 (from initial/hydride-shifted E1) + 4 (from methyl-shifted E1, first type) + 2 (from methyl-shifted E1, second type) = 18 products.
