Question
Question: How many non-equivalent hydrogens are there in aspirin?...
How many non-equivalent hydrogens are there in aspirin?
Solution
Equivalent hydrogen are the hydrogen atoms that are completely interchangeable as to their role in a molecule. For instance, consider a methane molecule, it consists of a carbon atom and 4 hydrogen atoms bonded to it. But during the formation of methanol, the 4th hydrogen atom is replaced by an oxygen atom, however the replaced hydrogen is still in the compound but is attached to the oxygen atom instead of the carbon atom.
Formulas used: No formulas will be used in the solution of the given problem.
Complete step-by-step answer: Aspirin as we know is used as a form of medication to reduce pain, fever or inflammation. Aspirin is a medically relevant and important drug for it is given shortly after a heart attack to decrease the chances of death. So, in a way aspirin is a life saver drug.
This life saver drug, aspirin has a complex chemical structure and is given by the IUPAC name acetylsalicylic acid or 2-Acetoxybenzoic acid. One way to identify the equivalent hydrogens of a molecule is to replace the hydrogen atoms with a different group of atoms and see if we get a different compound.
So, the structure of aspirin of 2-Acetoxybenzoic acid or C9H8O4 is:
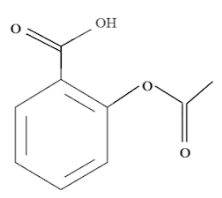
As we can see that the benzene ring usually has 6 hydrogen atoms and thus would be equivalent, but now, with the addition of a oic functional group and an acetyl substituent group, we have the following sets of equivalent hydrogen: CH3,COOH,H−3,H−4,H−5,H−6 .
They have 5 sets of different non-equivalent hydrogen which cannot be interchanged for any other functional group.
Note: The equivalence of hydrogen atoms in a compound are related and confirmed by the spectroscopy of these compounds.
