Question
Question: How many more grams of \[KI\] will dissolve in \(100g\) of water at \({{40}^{\circ }}C\) than at \({...
How many more grams of KI will dissolve in 100g of water at 40∘C than at 20∘C ?
Solution
Here the concept of solubility works, solubility is the amount of a particular solute to dissolve in a certain amount of solvent at that temperature. We know that the solubility increases as the temperature increases. The daily life example you can take is the making of sweet syrup for sweets. Here it was asked for the extra amount that we need at these two temperatures.
Complete step by step answer:
The solubility of different solutes can be seen by using the solubility curve. In the curve we have solubility in (100gcm3water)Vs(temperature) this graph shows how the solubility increases with the increase in temperature. So, see the plot for potassium iodide which is given below.
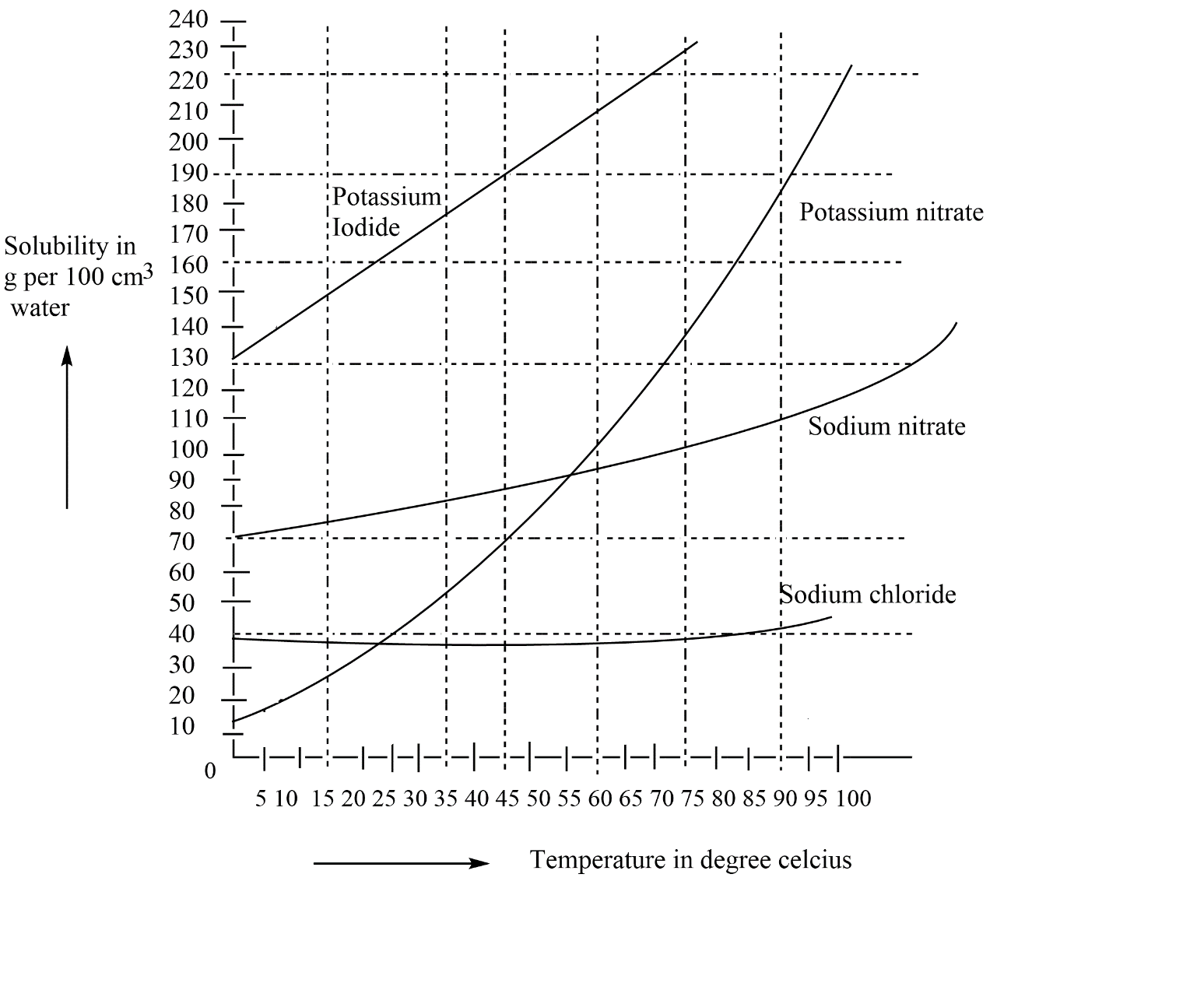
Here, as we are seeing the solubility of different salts in water and their changes with respect to temperature. So, now see for potassium iodide that at 40∘C the amount of potassium iodide dissolved in water is 195g you will able to see through the lines drawn so similarly of we see the solubility of same salt that is potassium iodide at 20∘C there we have the amount of potassium iodide as 155g . So, as asked in the question, the extra amount that we need for the two temperatures so we have to find out the difference in amount of potassium iodide at two different temperatures.
Amount of potassium iodide needed =195g−155g
=40g
Thus we need 40g grams of KIfor dissolving in 100g of water at two temperature differences.
Note: Saturated solution is formed for a particular temperature. So suppose we want a saturated solution of potassium iodide at 40∘C it means there is a particular amount of this alt which will dissolve in the water. The moment when no more potassium iodide will dissolve in some amount of water at that particular temperature it will be the saturation point.
