Question
Question: How many moles of iodomethane are consumed in the following conversion? $CH_3NH_2 \xrightarrow{CH_3...
How many moles of iodomethane are consumed in the following conversion?
CH3NH2CH3I(CH3)4N+I−
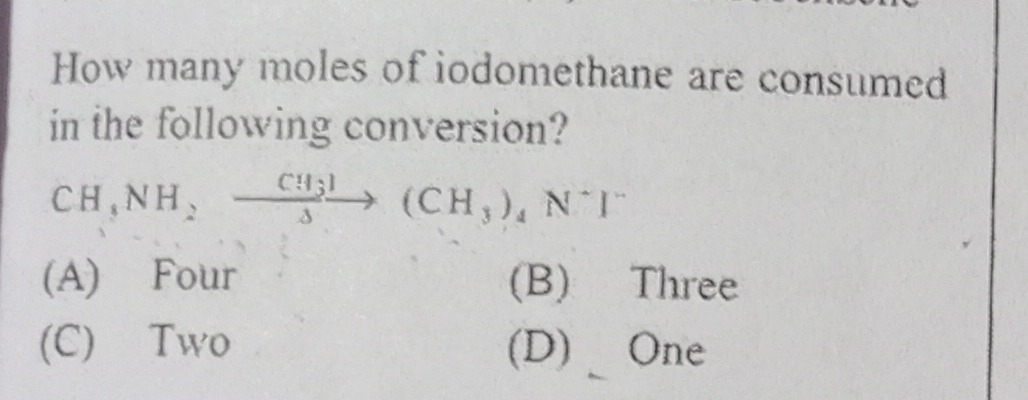
A
Four
B
Three
C
Two
D
One
Answer
Three
Explanation
Solution
Methylamine (CH₃NH₂) reacts with CH₃I in three successive alkylation steps:
-
CH3NH2+CH3I→CH3NHCH3+HI
-
CH3NHCH3+CH3I→(CH3)2NCH3+HI
-
(CH3)2NCH3+CH3I→(CH3)3NCH3+I−
Thus, a total of 3 moles of iodomethane (CH₃I) are required.
