Question
Question: How many moles of Grignard react with the following compound? 
Solution
Grignard reagent is an organomagnesium compound having a chemical formula R−Mg−X(where R is an alkyl or aryl group and X is a halogen). Different groups react differently with Grignard’s reagent. For reaction, only the functional group are going to react so the other groups for one particular reaction can be taken as R.
Complete answer:
The question says to find out the total number mole of Grignard reacting with the following compound. This means we have to individually find out how many moles of Grignard will be required for one group to react and then adding the individual moles will give us the total moles.
For this question, you should know how Grignard will react with different types of groups.
We have three different groups here which are CN, HS, CHO, so there will be three different reactions taking place. So let us see the reaction below along with the moles Grignard used.
(For every reaction we will only consider the main group which will react and the entire left out part will be written as R)
Our first reaction is with cyanide:
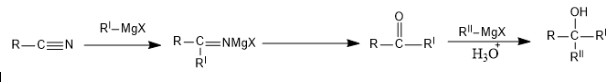
2 Moles of Grignard are used.
Next reaction is with aldehyde group:
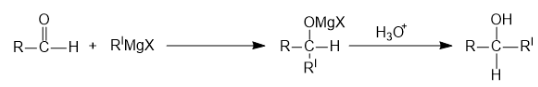
1 Mole of Grignard is used.
And the final reaction is with HS

1 Mole of Grignard is used.
Now just add up all the moles 2+1+1=4. And we see that in total 4 moles of Grignard is used.
Therefore the correct option is C. 4
Note:
In some questions, there might be more than 3 or 4 groups, all of those questions are to be done in the same manner. Grignard reagent does not react with a single halogen attached to benzene. The Grignard reagent is important for the formation of carbon-carbon bonds.
