Question
Question: How many moles of acetic anhydride \((A{c_2}O)\) is needed to react completely with sucrose : A. 8...
How many moles of acetic anhydride (Ac2O) is needed to react completely with sucrose :
A. 8
B. 6
C. 4
D. 5
Solution
:Sucrose is a disaccharide that is a carbohydrate which upon hydrolysis gives two molecules of the same or different monosaccharide . In case of sucrose , it gives D-glucose and D-fructose in equal amounts on hydrolysis .
Complete step by step answer:
Sucrose contains two monosaccharide units that are glucose and Fructose which are linked through their reducing centres . Enzymatic studies have revealed that C1−α of glucose is connected to C2−β of fructose. Further , the determination of ring size has revealed that in sucrose glucose is present in the pyranose form while Fructose is present in the furanose form . On the basis of the above information the structure of sucrose is as follows
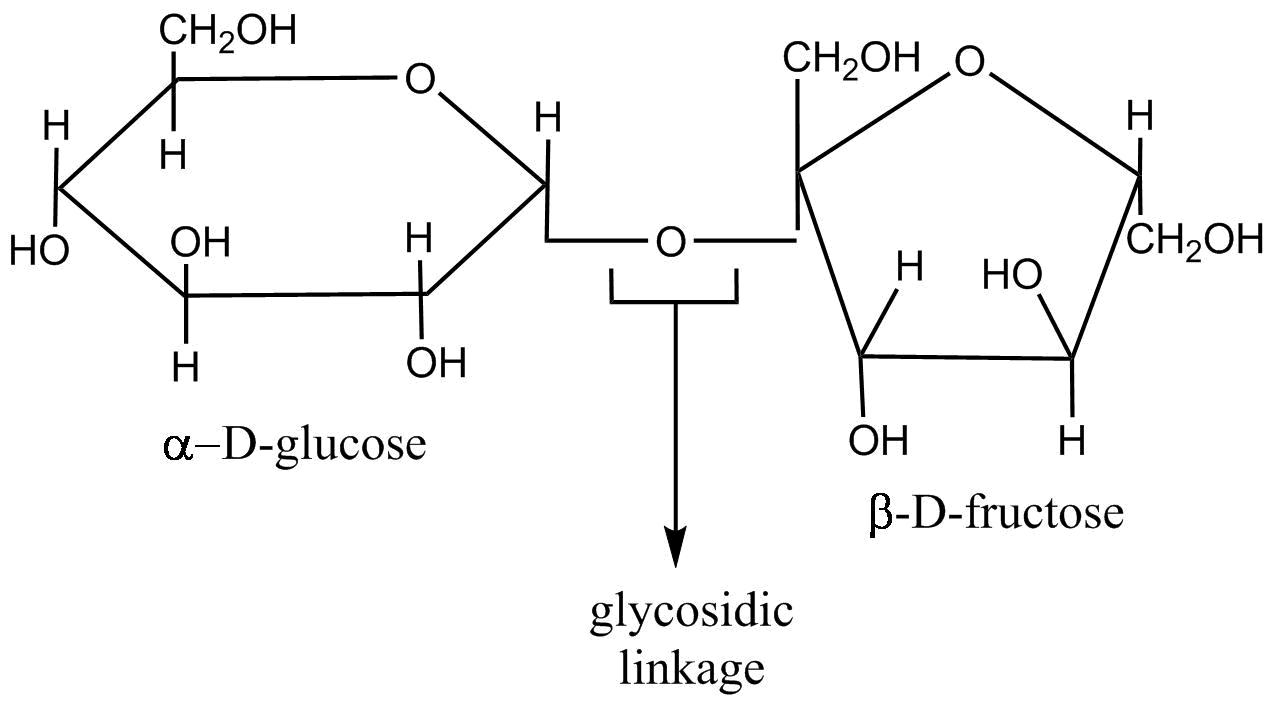
As you can see from the above structure that sucrose contains 8 OH groups , that is four OH groups from glucose and four from fructose .
Therefore when acetic anhydride reacts with sucrose , sucrose needs eight moles of acetic anhydride to react completely as there are eight OH groups .
So, the correct answer is Option A.
Note: As we know that glucose and fructose contain five OH groups , so sucrose should contain ten OH groups but actually it contains only eight OH groups . This is because when these two monosaccharides join to form sucrose there is loss of a water molecule ( so two OH groups are lost) and they are linked through oxide linkage which is also known as glycosidic linkage .
