Question
Question: How many moles of acetic anhydride \((A{c_2}O)\) is needed to react completely with sucrose? A.8 ...
How many moles of acetic anhydride (Ac2O) is needed to react completely with sucrose?
A.8
B.6
C.4
D.5
Solution
Sucrose contains 8 −OH groups and each of these −OH groups reacts with acetic anhydride.
If sucrose goes through acid catalysed hydrolysis, it produces one mole of D-Glucose and one mole of D-Fructose.
Complete step by step answer:
Sucrose is a disaccharide, a molecule that is formed when two monosaccharides are combined. It is produced naturally in plants like sugarcane, sugar beets, dates. Its molecular formula is C12H22O11
Sucrose (C12H22O11) has 8 −OH groups and each of these −OH groups will react with acetic anhydride. They react to form sucrose octa - acetate.
Sucrose + acetic anhydride 🡪 sucrose octa - acetate
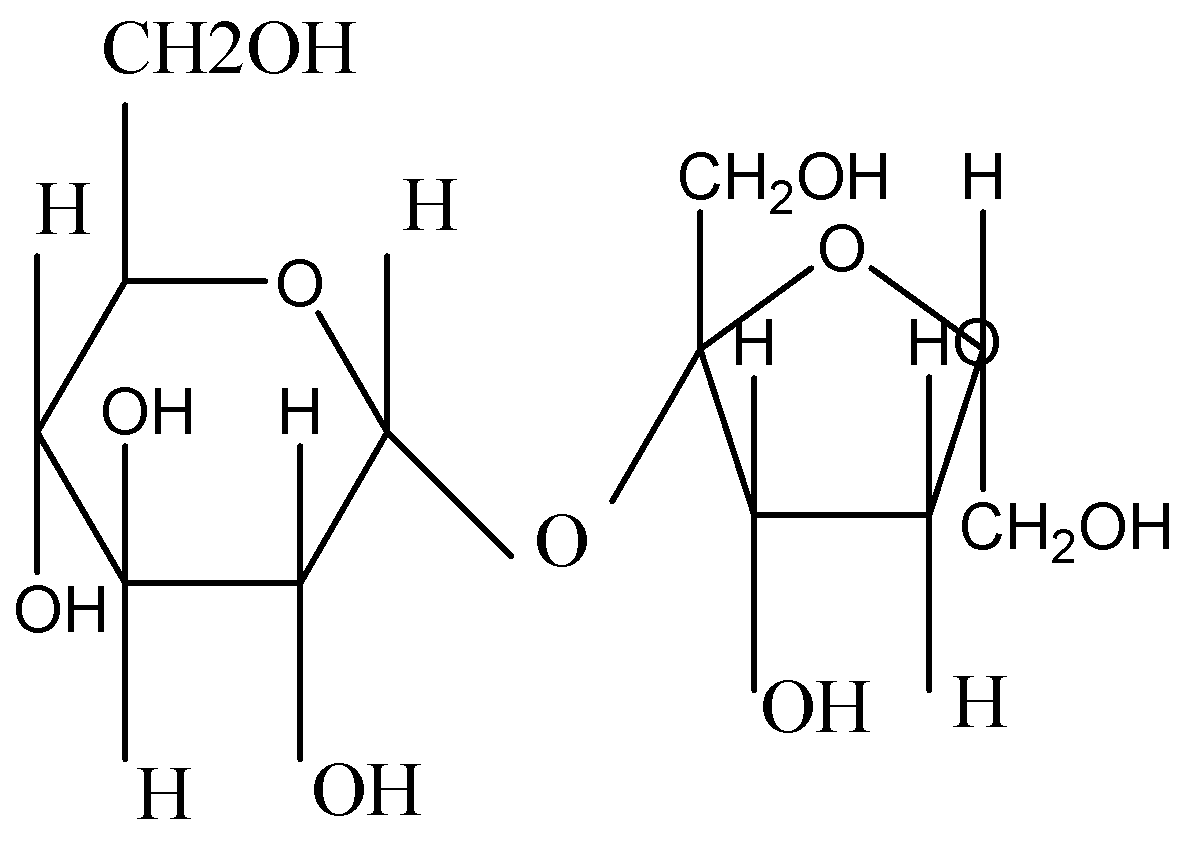
Figure: Sucrose
Therefore, 8 moles of acetic anhydride are needed to react completely with sucrose.
So, the correct answer is option (A).
Note: For every alcohol group present in the molecule, one mole of reactant i.e. acetic anhydride is needed.
Sucrose is the scientific name for ‘table sugar’ or ‘cane sugar’. It is an abundant disaccharide which contains 50% glucose and 50% fructose molecule. It is naturally found in many vegetables, fruits and grains. Plants use sucrose as the storage molecule. Its molar mass is 342 g/mol. It is the most common form of carbohydrate to transport carbon within plants. Almost all life on Earth is dependent upon sucrose and other carbs produced by the plants. It was one of the very first substances to be extracted from the plants on a large scale, creating the white sugar table that we know today.
