Question
Question: How many minimum carbons are required for chain isomerism and position isomerism in alkynes?...
How many minimum carbons are required for chain isomerism and position isomerism in alkynes?
Solution
The chain isomerism is the isomerism in which there is a difference in arrangement of the parent carbon chain but has the same molecular formulae.
- Position isomerism is the isomerism shown by the compounds which possess multiple bonds or having a functional group or substituents attached to the carbon atoms.
Complete Solution :
So in the question we are asked to comment on how many carbon atoms are required for an alkyne to show the chain isomers and positional isomerism.
- We have studied the basics of isomerism and its types in the organic chemistry of lower classes. Now let’s brush up our memory about isomers.
- Isomers are those molecules which possess the same molecular formula but differ in the arrangement of the carbon chains, substituents attached to the carbon chain which refers to the structural isomers and differ in the arrangement of atoms or molecules in space which comes under the classification of stereoisomers.
- Even though the molecules are having the same molecular formulae they differ in their physical and chemical properties i.e. isomers do not possess similar properties.
- Chain isomerism is also known as skeletal isomerism which comes under the classification of structural isomers . It is the isomerism in which the two molecules that have the same formulae differ in the parent carbon skeleton i.e. the two compounds consist of different parent carbon chains. Commonly, chain isomers differ in the branching of the carbons.
Now let’s see how many carbons are required for an alkyne to show chain isomerism.
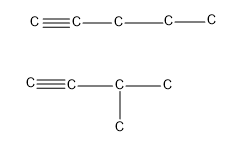
- Alkynes are molecules in which a carbon contains a triple bond. We can draw the structure and let’s see which carbon chain will give chain isomerism.
- So the first alkyne to show chain isomerism will be pentyne i.e. carbon chain with five carbons.
Hence at least five carbons should be present in an alkyne to show chain isomerism.
- In position isomerism, the molecules will have the same molecular formula but the positions of the functional groups or substituent atoms are different. Typically, this isomerism involves the attachment of the functional groups or presence of multiple bonds in different carbon atoms in the carbon chain.
Now let’s see how much carbon atoms are required for an alkyne to show position isomers.
- The first alkyne in the homologous series that can show position isomerism is butyne i.e. an alkyne requires at least four carbon atoms to show position isomerism.
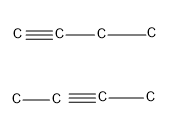
Therefore, the minimum number of carbon atoms required for chain isomerism in alkynes are 5 and the minimum number of carbon atoms required for position isomerism in alkynes are 4.
Note: Remember that the answer for the same question would be different in case of alkanes and alkenes. The triple bond in alkynes prevents the carbon from forming more bonds. So more carbons are required in that case to exhibit chain isomerism.
