Question
Question: How many maximum electrons can be filled in p-orbital?...
How many maximum electrons can be filled in p-orbital?
Solution
Every orbital has a shape in which the probability of finding the electron is the most. We know that for p-orbital, the azimuthal quantum number is 1, so the magnetic quantum number will be 3 (-1, 0, +1). And each magnetic quantum number can accommodate two electrons that have opposite spin.
Complete step-by-step answer: There are four quantum numbers that can help to find the number of electrons in a shell or any orbital. First, the principle quantum number tells the number of shells and is shown by n. Second, the azimuthal quantum number tells the number of sub-shell and the magnetic quantum number tells the number of orbitals and each orbital can have two electrons having an opposite spin. No orbital can have two electrons having the same spin.
Till now we now 6 shells having the p-orbital, i.e., 2 – 7. These are written as 2p,3p,4p,5p,6p, and 7p. We know that for p-orbital, the azimuthal quantum number is 1, so the magnetic quantum number will be 3 (-1, 0, +1). So, there are three orbitals of the p-subshell. These are represented as:
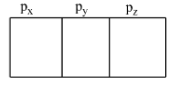
So, each of the orbitals can accommodate a maximum of two electrons and these must have opposite spin. Therefore, there are three orbitals, hence there can be a total of six electrons and this is shown below:
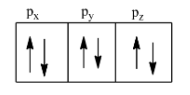
Note: By studying these quantum numbers we came to know that the s-orbital can have two electrons, the d-orbital can have 10 electrons, and the f-orbital can have 14 electrons.
