Question
Question: How many lone pairs are on the central atom in the perchlorate ion?...
How many lone pairs are on the central atom in the perchlorate ion?
Solution
This question is based on the concept of lone pair and hybridization. To answer such types of questions firstly we need to find out its hybridization and its shape and then we can easily find the lone pairs of electrons.
Complete step-by-step answer:
The given ion is the perchlorate ion (ClO4−)
The formula for the steric number of the given ion can be written as,
Steric number = 21[V+N−C+A]
In the above formula,
V= number of valence electron present in the central atom
N= number of monovalent atoms (having valency 1) bonded to central metal atoms.
C= charge of cation
A= charge of anion
Therefore, number of electrons in the ClO4−chlorate ion is
In this question for the given ion V=7 (as chlorine has 7 valence electrons) and A=1 (one negative charge). There are no monovalent atom and cation so N=C=0)
Therefore, steric number= 21[7+1]=4electrons
The steric number is 4, that means the hybridization of the given ion will be sp3and thus we can that the geometry of the molecule will be tetrahedral
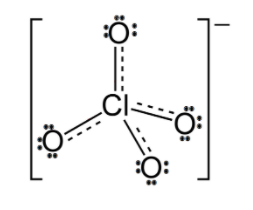
We already know the fact that the number of valence electrons present in chlorine atoms is 7. Since, the charge of the molecule is -1. One more electron is added to the compound. Now, the total number of electrons in the compound has become eight. In the figure, all eight electrons are used in the formation of covalent bonds with the oxygen. Hence, there are no electrons left unshared or in other words we can say that there are zero lone pairs of electrons.
Note: Always find the lone pairs by drawing the structure of a given molecule. Students should be careful that in this question we were asked about lone pairs of chlorine atoms only and hence it was zero. If the lone pairs were asked for the entire compound, then the nine lone pairs of oxygen would be also counted.
