Question
Question: How many lone pairs are on the Br atom in \(BrF_{2}^{-}\) ? A. 2 B. 3 C. 0 D. 1...
How many lone pairs are on the Br atom in BrF2− ?
A. 2
B. 3
C. 0
D. 1
Solution
The electron pair which are not involved in bonding with other atoms and are present in outermost orbitals are called lone pairs of electrons. If we know the Lewis dot structure of the compound we can easily calculate the number of lone pairs of electrons.
Complete answer:
- In the question it is asked to calculate the lone pair of electrons on a bromine atom in BrF2− molecule.
- To know about the lone pair of electrons in BrF2− we should know about the Lewis dot structure of the BrF3 and it is as follows.
- The Lewis dot structure of BrF3 is as follows.
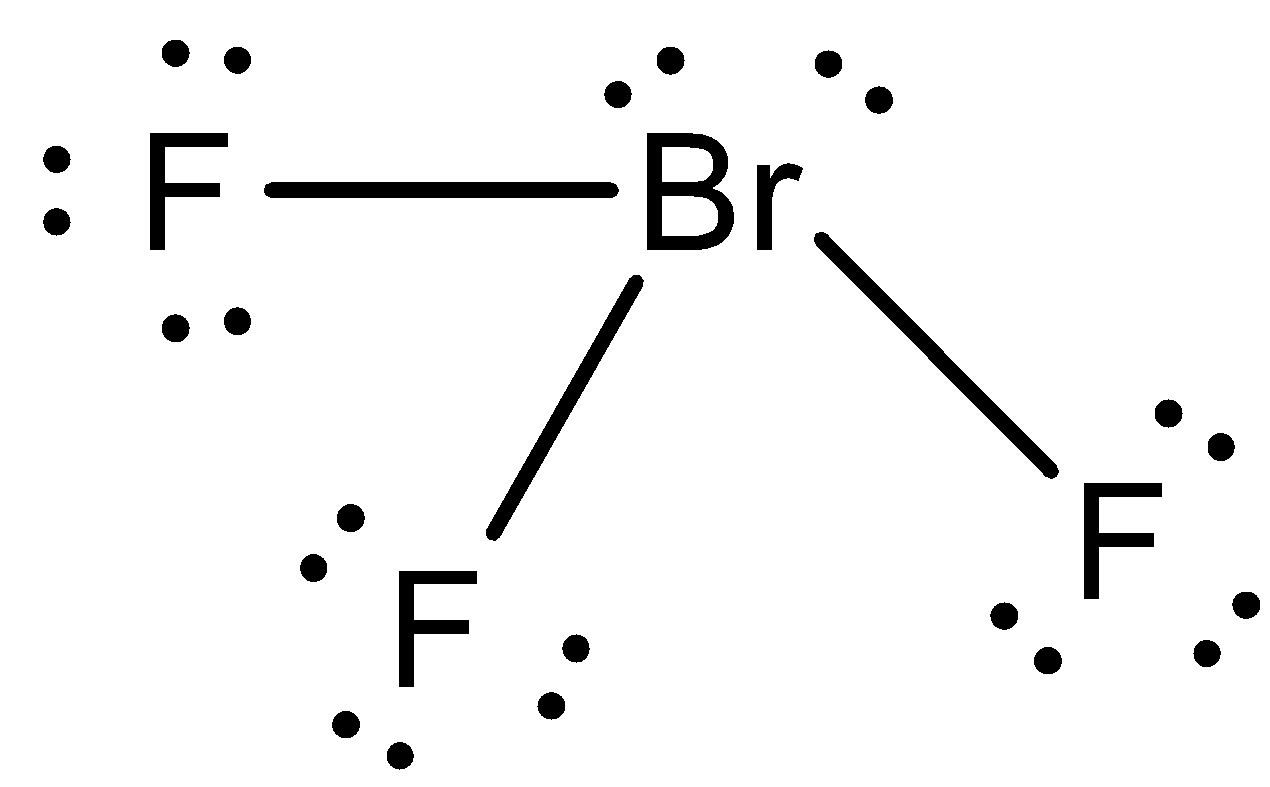
- In the above Lewis dot structure there are three lone pairs of electrons on each fluorine atom and two lone pairs of electrons on the bromine atom.
- The Lewis dot structure of the BrF2− molecule is going to formed by removing one fluorine atom from BrF3 molecule and it is as follows.
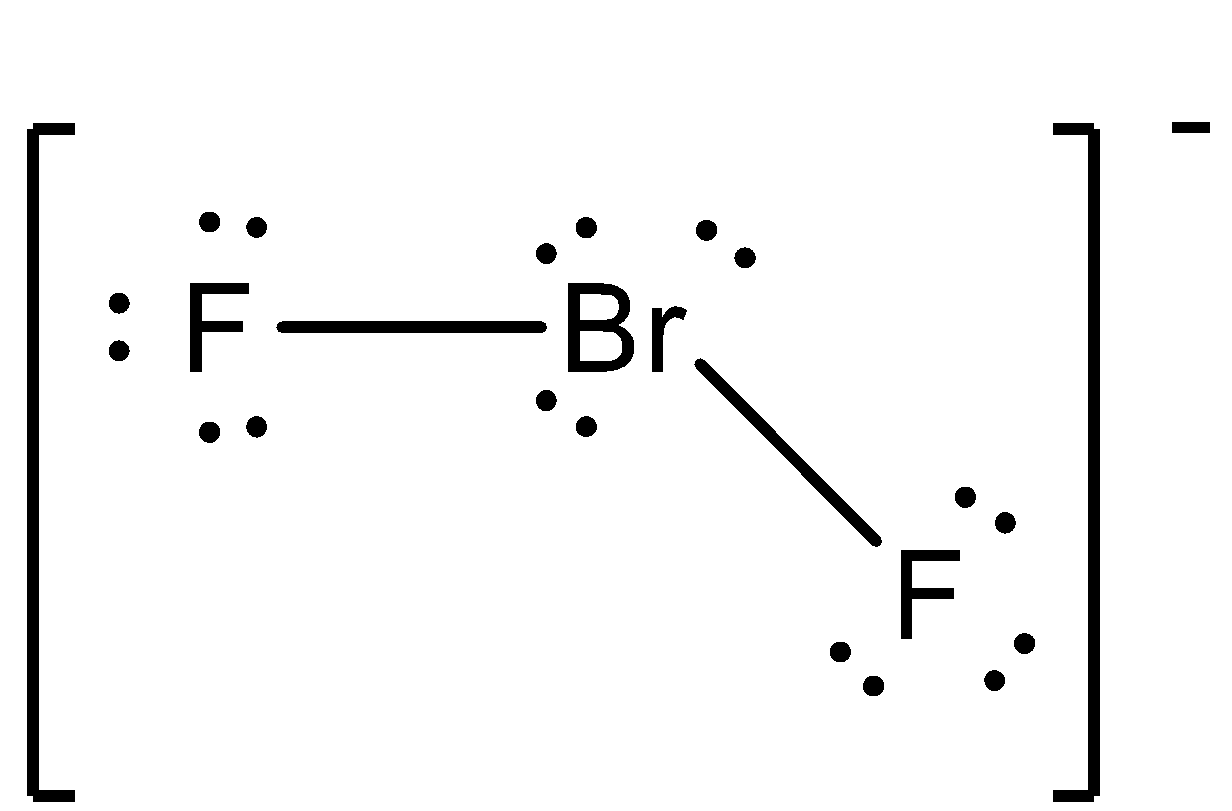
- From the above Lewis dot structure we can easily say that each fluorine atom has three lone pairs of electrons.
- The bromine atom has three lone pairs of electrons.
- Out of those three lone pair electrons two lone pairs are naturally present and the third lone pair electrons come on the bromine atom due to removal of a fluorine atom from BrF3 .
Therefore the correct option is, ‘B. 3’.
Note: The BrF2− molecule is not stable. BrF3 and BrF5 are the stable derivatives of the bromine. The number of valence electrons in BrF2− are 22. Out of those 22 lone pairs of electrons 20 pairs are involved in bonding.
