Question
Question: How many ketones are possible with the molecular formula\[{C_6}{H_{12}}O\] ? A) \[5\] B) \[6\] ...
How many ketones are possible with the molecular formulaC6H12O ?
A) 5
B) 6
C) 7
D) 8
Solution
To answer the question we need to know how we can draw structures from a given molecular formula. Utilizing evenness, and the way that it should be a ketone, the carbonyl group can just move between carbons 2 and3. That makes for the initial two isomers. Two additional isomers include moving the terminal methyl bunch up one to carbon4. Two additional isomers include moving the terminal methyl bunch up one to carbon4. The fifth and 6th isomers are enantiomers, the highest point of which is R and the lower part of which isS. The thought was to move the methyl bunch up one more to carbon3, making a sec-butyl bunch off of(CH3)2CO. The seventh isomer was essentially to make a tert-butyl bunch hanging off of(CH3)2CO.
Complete step-by-step answer:
The simplest complex ketone isCH3−C(=O)−CH3. Its atomic formula isC3H6O. From this formula, we can say that for "n" carbon iotas we need "2n" hydrogen molecules and an oxygen particle. Henceforth the overall recipe of the ketone isCnH2nO.
Keto group contains a carboxyl gathering which has two alkyl bunches connected to it each on one or the other side. Primary formula: R−C=O−R1. Aldehydes and ketones are natural mixes that join a carbonyl functional group,C=O.
We can write seven ketone structures with the molecular formulaC6H12O.
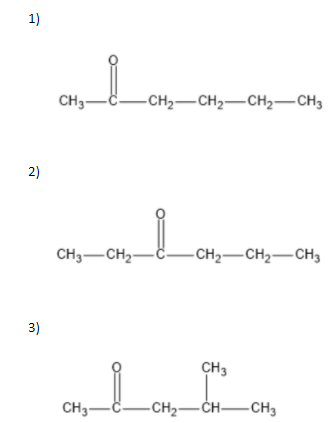
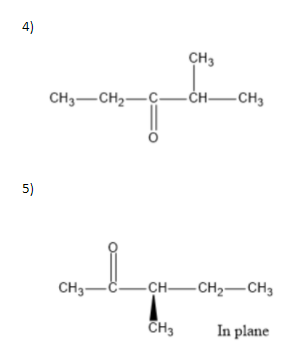
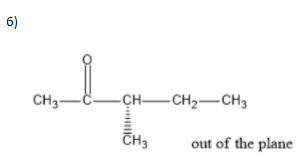
7)
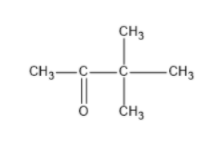
Note: We know that a carbonyl group can only exist at spots that give a sp2 hybridization, so there is no structure for the carbonyl on carbon 3 when carbon 3 has a tert-butyl group. Similarly, there is no structure for which the carbonyl is on carbon 3 while the sec-butyl is also on carbon3.
