Question
Question: How many isomers of molecular formula \({{C}_{3}}{{H}_{9}}N\) will liberate \({{N}_{2}}\) gas on tre...
How many isomers of molecular formula C3H9N will liberate N2 gas on treatment with HNO2 .
a.) One
b.) Three
c.) Two
d.) Five
Solution
Isomers are defined as the molecules which have the same molecular formula but different molecular geometries. There are two types of isomers such as conformational isomers and constitutional isomers.
Complete Solution :
In the given formulae C3H9N, four isomers are possible. They are propan-1-amine, propan-2-amine, N-methylethanamine, N,N-Dimethylmethanamine. The structure of different isomers which can be drawn corresponding to the given molecular formula are given below:
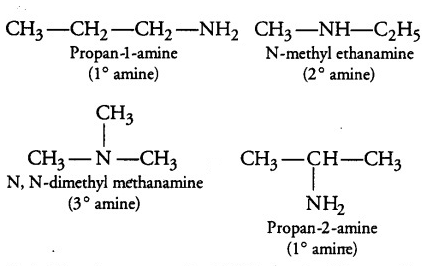
- Among the given isomers of C3H9N only primary amines will liberate nitrogen gas when it will be treated with nitrous acid i.e. propan-2-amine and propan-1-amine will liberate nitrogen gas when it will be treated with nitrous acid. The reactions involved are mentioned below:
& \underset{propan-1-amine}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}+HN{{O}_{3}}\to }}\,C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH+{{N}_{2}}+HCl \\\ & \underset{propan-2-amine}{\mathop{C{{H}_{3}}CH(N{{H}_{2}})C{{H}_{3}}+HN{{O}_{3}}\to }}\,C{{H}_{3}}CH(OH)C{{H}_{3}}+{{N}_{2}}+HCl \\\ \end{aligned}$$ **So, the correct answer is “Option C”.** **Additional information:** We know that there is no superimposable mirror image and there is no plane of symmetry in the molecule then the molecule is known as chiral. And the chiral carbon has four different groups attached to the carbon. This property is known as chirality and the compounds which have the same molecular formula but different compounds are said to be isomers. The compounds which are mirror images but are not identical to each other. They are known as enantiomers. **Note:** Structural isomers are defined as compounds which have the same molecular formula but different connectivity of atoms or bonds. The isomers which are different by the orientation of atoms in space are known as stereoisomers and the isomers which differ by their rotation around a single bond are known as conformational isomers.