Question
Question: How many isomers of \({{C}_{4}}{{H}_{10}}O\) react with \(C{{H}_{3}}MgBr\) to evolve \(C{{H}_{4}}\) ...
How many isomers of C4H10O react with CH3MgBr to evolve CH4 gas? (Excluding stereoisomer)
Solution
Grignard’s reagents RMgX are very reactive organometallic compounds with carbon-metal bonds. They can react with acidic hydrogen of alcohols to give corresponding hydrocarbons. A general chemical equation for reaction between alcohol and Grignard reagent is
Rδ−−Mgδ+Xδ−+ROδ−−Hδ+→R−H+Mg(OR)X
Complete answer:
Let us first find the total number of isomers possible with the chemical formula given, i.e. C4H10O
General formulas for alcohols and ethers can be given as CnHn+2O. The chemical formula given to us C4H10O fits the general chemical formula for both alcohols and ethers as C4H2×4+2O, therefore, we can say the C4H10O is an alcohol or ether.
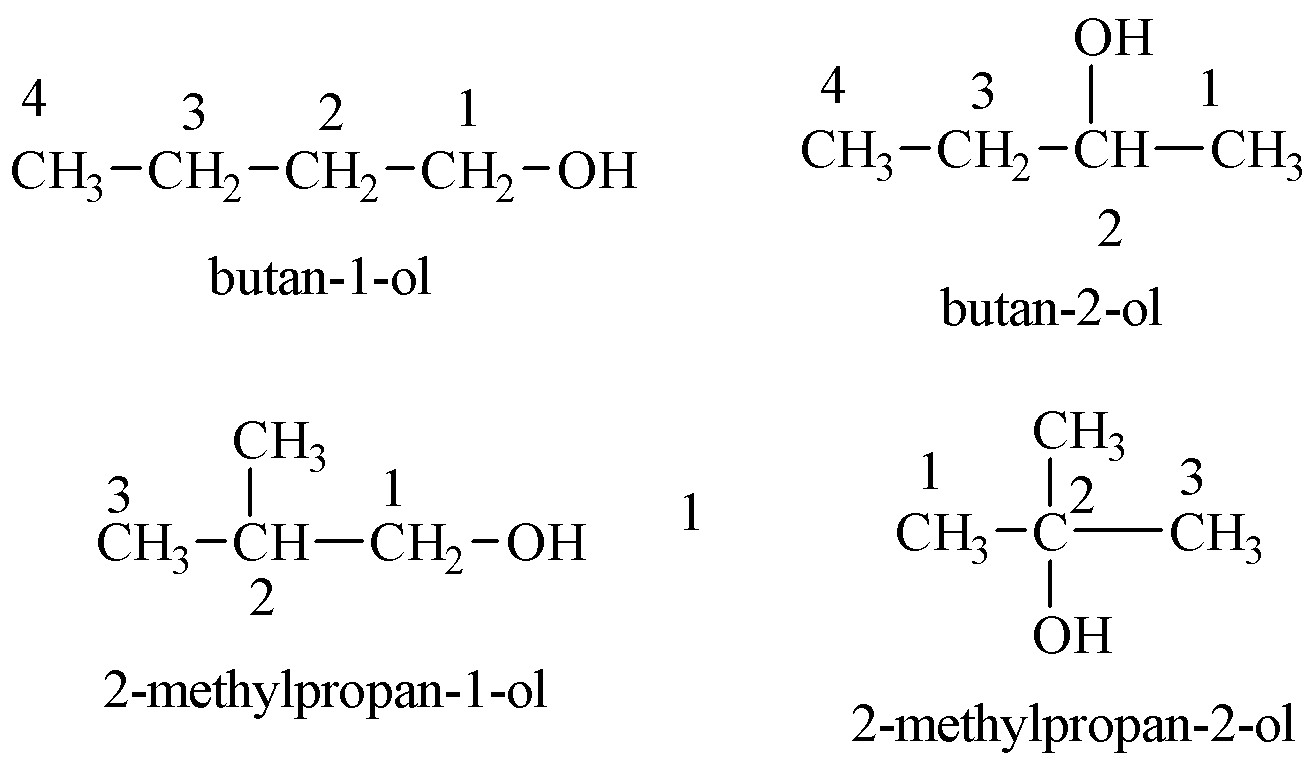
The possible structures of alcohols with C4H10O are shown below:
Structures of ethers possible with the chemical formula are given below:
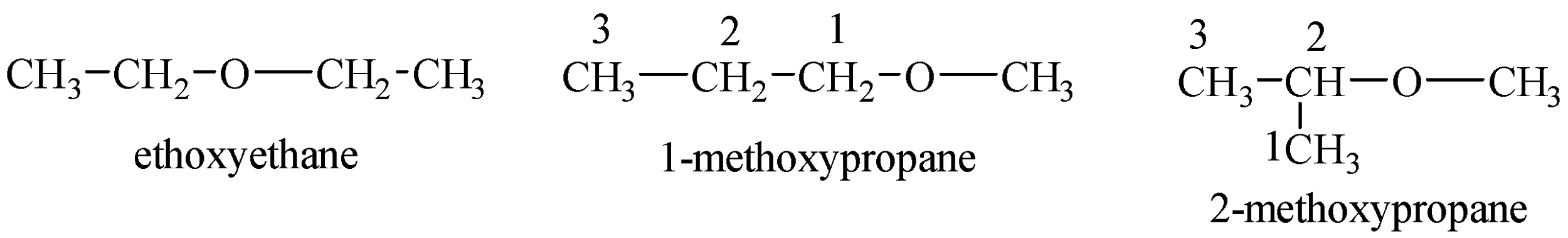
Now, we can see that ethers do not contain any acidic hydrogen and cannot give protons to Grignard’s reagent which is CH3MgBr in the given question.
Alcohols are acidic in nature due to the polar O−H bond, therefore, can react with Grignard’s reagent CH3MgBr to give release (CH4) gas. Reactions of all the four alcohols with CH3MgBr are given below:
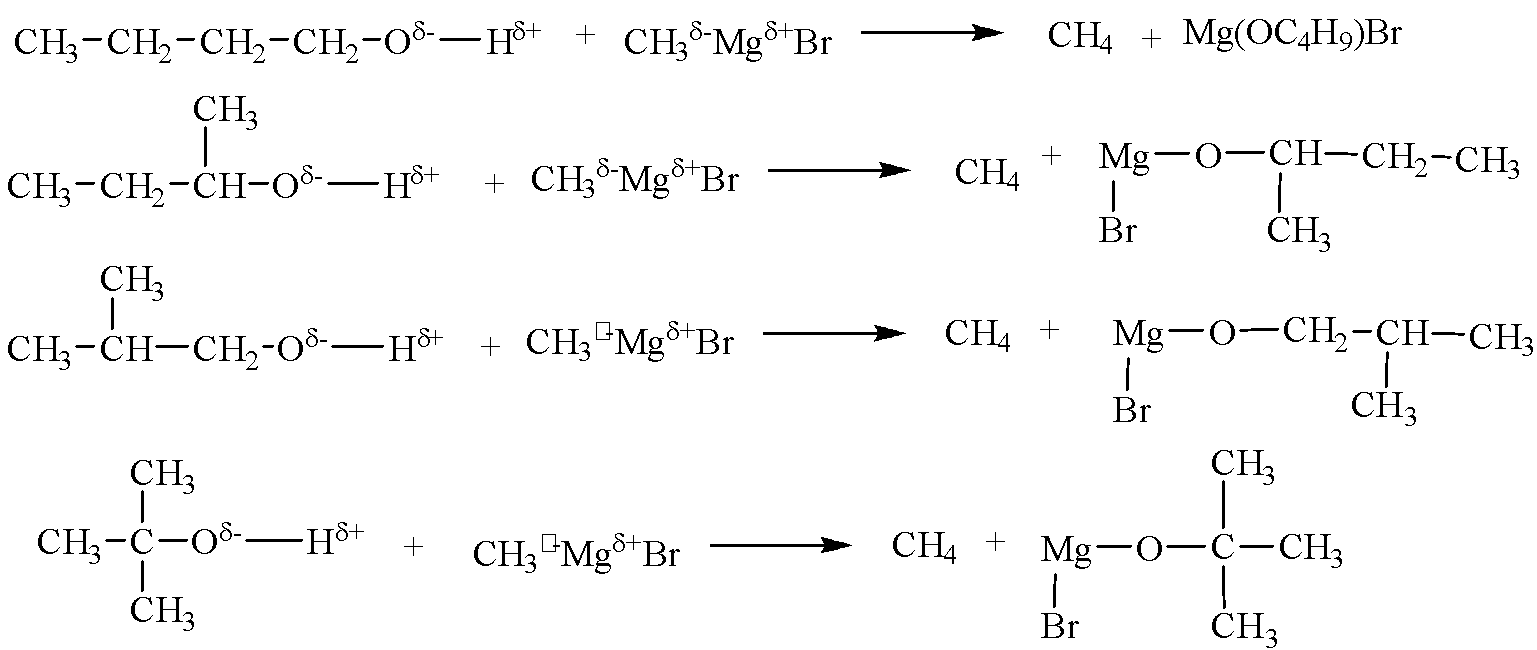
Therefore, four out of the seven isomers of C4H10O react with CH3MgBr to evolve CH4 gas.
So, the correct answer is “Option B”.
Additional Information: One stereoisomer of butan-1-ol is possible as it has a chiral carbon. Stereoisomers have the same molecular and structural formula, i.e. have connectivity of atoms but different spatial arrangement of atoms.
Note: In order to react with CH3MgBr, isomers of C4H10O must have an acidic hydrogen. Ethers do not contain any acidic hydrogen as the electronegative O is connected to an alkyl chain, hence, they do not react with Grignard’s reagent.
