Question
Question: How many isomers are possible for \({C_6}{H_{12}}\)? A: \(25\) B: \(24\) C: \(22\) D: None o...
How many isomers are possible for C6H12?
A: 25
B: 24
C: 22
D: None of these
Solution
Isomers are the molecules or ions that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. Isomers may or may not have the same physical and chemical properties.
Complete step by step answer:
Isomers are the molecules or ions that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. In C6H12 there are six atoms of carbon and twelve atoms of hydrogen. The possible structures of this compound are as follows:
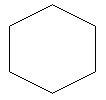
1. Cyclohexane: this is a cyclic hydrocarbon in which carbon atoms are arranged in the form of a ring.
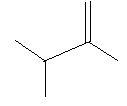
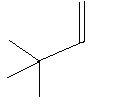
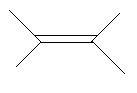
In the following three compounds there is a double bond between two carbon atoms in the main chain and two methyl groups are also present in the main chain.
2. 2,3−Dimethyl−1−butene
3. 3,3−Dimethyl−1−butene
4. 2,3−Dimethyl−2−butene
In the following three compounds there is a ring of four carbon atoms and two methyl groups are present at different positions.
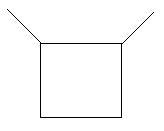
5. 1,1−Dimethylcyclobutane
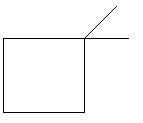
6. 1,2−Dimethylcyclobutane
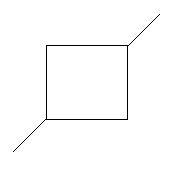
7. 1,3−Dimethylcyclobutane
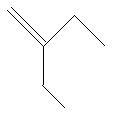
8. 2−Ethyl−1−butene: in this compound there are four carbon atoms in the main chain with a double bond at first carbon atom and one ethyl group is present at second carbon atom.
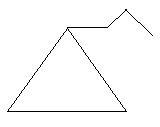
9. Ethylcyclobutane: in this compound there is a ring of four carbon atoms and one ethyl group is present as the first carbon atom of the ring.
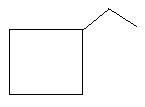
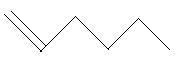
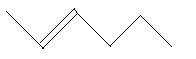
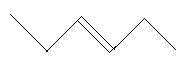
In the following three compounds, there is a main chain of six carbon atoms and one double bond is present at different positions.
10. 1−Hexene
11. 2−Hexene
12. 3−Hexene
In the following six compounds there is a main chain of five carbon atoms in which methyl groups and position of double bond changes.
13. 2−Methyl−1−pentene
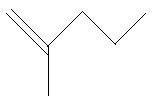
14. 2−Methyl−2−pentene
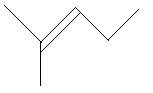
15. 3−Methyl−1−pentene
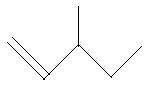
16. 3−Methyl−2−pentene
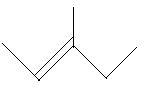
17. 4−Methyl−1−pentene
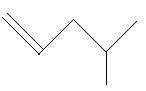
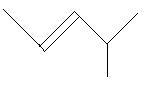
18. 4−Methyl−2−pentene
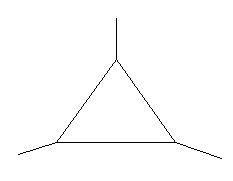
In the following two compounds there is a ring of three carbon atoms and three methyl groups are present at three different positions.
19. 1,2,3−Trimethylcyclopropane
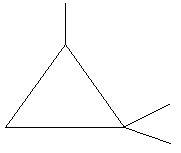
20. 1,1,2−Trimethylcyclopropane
21. Propylcyclopropane: in this compound there is a ring of three carbon atoms and a side chain of three carbon atoms is also present.
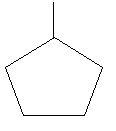
22. Methylcyclopentane: in this compound there is a ring of five carbon atoms and one methyl group is attached to this ring.
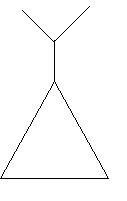
23. Isopropylcyclopropane:
In the following two compounds there is a ring of three carbon atoms and the position of one ethyl and methyl group varies.
24. 1−Ethyl−1−methylcyclopropane
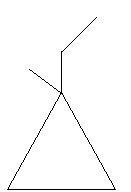
25. 1−Ethyl−2−methylcyclopropane
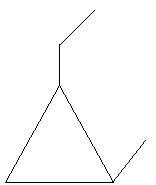
So, there are total 25 isomers of C6H12.
Hence the correct option is A.
Note:
In these isomers geometric isomers and enantiomers are not included. Enantiomers are the non-superimposable images of each other. Enantiomer is one of the two stereoisomers. Stereoisomers are the isomers which differ in three dimensional arrangement.
