Question
Question: How many isomeric vicinal-dihalides are possible for the compound having a molecular formula \[{C_3}...
How many isomeric vicinal-dihalides are possible for the compound having a molecular formula C3H6Cl2?
A.1
B.2
C.3
D.4
Solution
Vicinal compounds have halogens on adjacent carbons. Elaborate the molecular formula to carefully look at the structure and then decide the correct answer to the problem.
Complete answer:
In the question it is asked to identify how many isomeric vicinal-dihalides are possible for the compound C3H6Cl2. For this you should know what vicinal dihalides are. So vicinal dihalides are those compounds which have halogens present on the adjacent carbons. The vicinal dihalides are obtained as a result of two successive elimination reactions.
Now we will look into the structure from the molecular formula:C3H6Cl2 The structure is:
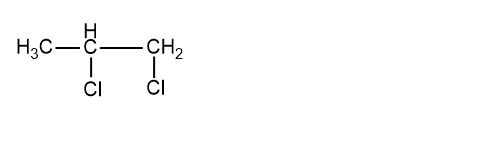
The name of the above compound is 1, 2-dichloropropane. In this structure, we can see that two adjacent carbons are having Cl halogen attach to them so this is a vicinal dihalide. Let us now try to make an isomer of this compound to see whether it is possible or not. So the isomer of this is:
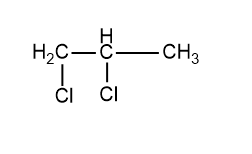
This is the isomer that we get but the name of this compound is also 1,2-dichloropropane, both the compounds drawn above and below are the same. No more isomeric vicinal halides can be drawn for this compound. Hence only 1 isomeric vicinal dihalide is possible for the molecular formula C3H6Cl2
Therefore the correct option A. 1.
Note:
There are also geminal-dihalides. These compounds have halogens present on the same carbon. They also are the result of two successive elimination reactions. Both vicinal-dihalide and geminal-dihalides are important concepts in chemistry.
