Question
Question: How many isomeric pentynes \(\left( {{C_5}{H_8}} \right)\) are possible: A. \(3\) B. \(4\) C. ...
How many isomeric pentynes (C5H8) are possible:
A. 3
B. 4
C. 5
D. 6
Given: The formula of the organic compound, pentynes is: (C5H8)
Solution
We can write the structures of different isomers by using different carbon skeletons keeping the same formula.
Complete step by step solution:
We know that isomers are those compounds that have the same molecular formula but different properties. In organic compounds, we have basically two types of isomerism: structural isomers and stereo-isomers. Here, we will discuss the structural isomerism that arises with difference in the structure. We can further classify structural isomers as follows:
- Chain isomers: these have different carbon skeletons
- Position isomers: these have functional group/substituent positioned differently
- Functional group isomers: these have different functional groups
- Metamers: these have different alkyl chains at the sides of a functional group
We are given the organic compound, pentyne whose chemical formula is C5H8. let’s try to draw structural isomers with formula C5H8 as follows:
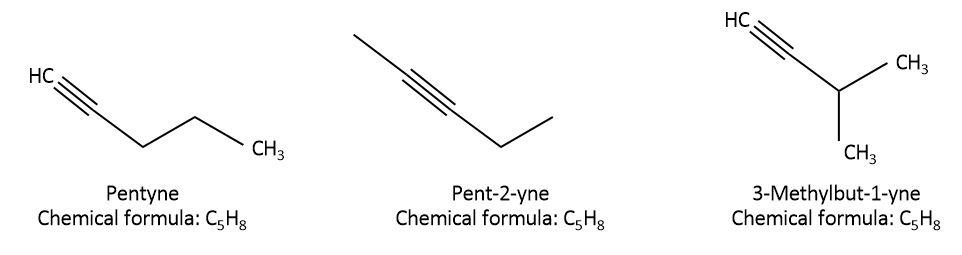
As we can see that in pentyne, all the 5 carbons are attached in a straight chain and the triple bond is between the first and second carbon. This can also be called n-pentyne or pent−1−yne. In the second isomer, pent−2−yne, all the 5 carbons are attached in a straight chain but the triple bond is between second and third carbon. In the last isomer, 3−methylbut−1−yne , only 4 carbons are attached in a straight chain, triple bond is between first and second carbon and there is one methyl group present as a substituent on the third carbon. So, all the three isomers have different structures but the molecular formula is the same for all of them.
**Hence, the correct option is A.
Note:**
We can see that positioning the triple bond between third and fourth or fourth and fifth carbons would have the same structure as that of pent−2−yne and pent−1−yne respectively.
