Question
Chemistry Question on coordination compounds
How many hydrogen bonded water molecule (s) are associated with CuSO4⋅5H2O ?
1
2
3
4
1
Solution
The structure of complex CuSO4,5H2O or Cu(H2O)SO44H2O is as
In crystalline form, four water molecules are coordinated with Cu atom forming a square-planar geometry and the two O atoms of sulphate ion complete the distorted octahedron. The fifth water molecule is attached through H-bonding between one of the coordinated H2O molecule and one of the sulphate ion
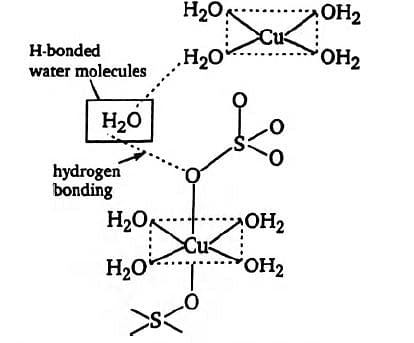
The geometry of copper (II) in copper sulphate pentahydrate is deformed octahedral.
The copper is connected to two oxygen atoms from two sulphate ions and four water molecules in a square-planar geometry, as can be seen in the structure. Another way to put it is that one H2O molecule is H-Bonded to sulphate ions in this situation.
Therefore, four water molecules are in coordination with the Cu2+ ion, while the fifth water molecule is hydrogen-bonded to the oxygen of the sulphate ion. Last but not least, the fifth water molecule is not coordinated, has a hydrogen bond, and is firmly entrenched in a crystal.
Thus, we may say that just four water molecules are coordinated, and the lone hydrogen bond is between the fifth and sixth molecules.
