Question
Question: How many hydrogen-bonded molecules are associated in \(CuS{{O}_{4}}.5{{H}_{2}}O\)....
How many hydrogen-bonded molecules are associated in CuSO4.5H2O.
Solution
Before solving this question, we have to first know what is Hydrogen bonding and then we can easily answer the question. In CuSO4.5H2O, There is H-bonding due to the presence of Oxygen between sulphate ions and water molecules.
Complete answer:
Hydrogen- Bonding occurs because of the dipole-dipole interaction between the Hydrogen atom and a strongly electronegative element and another electronegative atom lies in the region of the Hydrogen atom having lone pair of electrons.
CuSO4.5H2Ois known as Copper (II) sulphate pentahydrate. It has Copper(II) and two sulphate ions in the geometry. There are about five water molecules associated with it. Four water molecules are coordinated with Cu whereas there is only one water molecule that is hydrogen-bonded that too with SO4.
SO4. H2O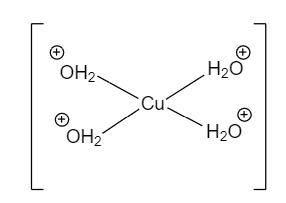
In this structure, There is coordinate bonding between Copper and Water molecules and coordinate covalent bonding between oxygen atoms.
So, there is only one hydrogen-bonded molecule in CuSO4.5H2O
Additional information:
Copper sulphate can be used as a fungicide as it can kill fungi. It is also used for blood tests as it detects anaemia. It can be used in vegetable dye.
Note:
Copper sulphate has a molecular weight of 249.69 g/mol is blue and is present in the crystalline form. It is odourless and has a metallic taste. The boiling point of it is 1207∘Fand the melting point is 297∘F. It is soluble in methanol and water. Stability is not definite when kept dry but it shows stability towards heat, cold and light. It is corrosive to steel. The pH of it is 4 and the density is 2.284.
