Question
Question: How many hydrogen atoms are in 5 molecules of isopropyl alcohol, \( {C_3}{H_7}O \) ?...
How many hydrogen atoms are in 5 molecules of isopropyl alcohol, C3H7O ?
Solution
The mole concept is very significant and useful in chemistry. It is actually the base of stoichiometry and it provides the best option to express the amounts of reactants as well as products that are consumed and formed during a chemical reaction.
Complete answer:
First of all the chemical formula given in question for isopropyl alcohol is wrong as the correct formula is C3H7OH not C3H7O . The structure for isopropyl alcohol is shown below:
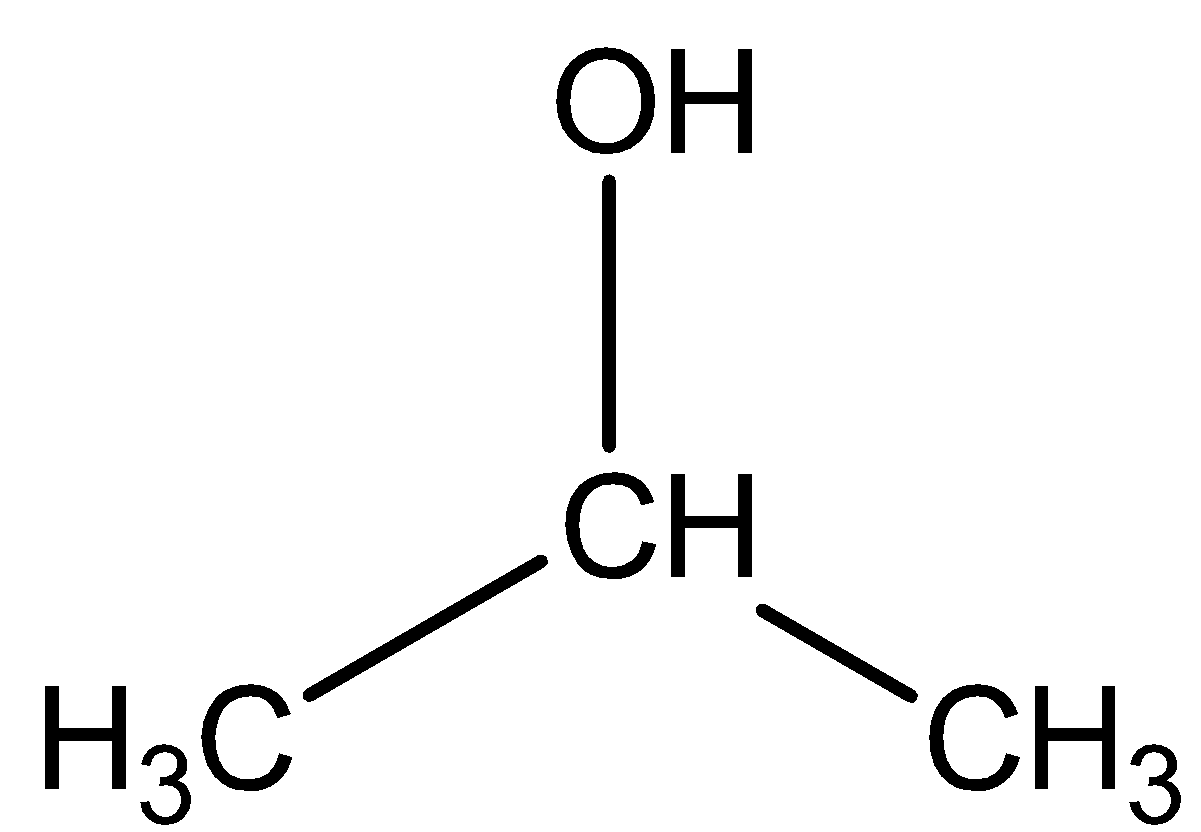
We know that C3H7OH actually represents one molecule of the compound i.e. isopropyl alcohol. It is clear from the chemical formula as well as from its chemical structure that one molecule of isopropyl alcohol comprises 8 Hydrogen atoms.
So applying the unitary method, 5 molecules of isopropyl alcohol will comprise Hydrogen atoms i.e. 8×5=40 .
Hence, 40 hydrogen atoms are in 5 molecules of isopropyl alcohol, C3H7OH .
Additional information: Avogadro's number was actually obtained by dividing charge of one mole of electron by the charge of one single electron that equals 6.02214154×1023 particles per mole. 1 mole of any substance contains 6.023×1023molecules
Thus, the number of molecules of any substance can be identified by multiplying the number of moles with Avogadro's number i.e. 6.023×1023 . We can say:
Number of molecules=number of moles×6.023×1023
Note:
If we are provided with the given mass of the compound, we can calculate molecular mass of that compound by adding the relative atomic masses of each element present in that particular compound. Then using these two values, we can calculate the number of moles. Then from the number of moles, we can calculate the number of molecules.
