Question
Question: How many geometrical isomers are possible for the given molecule? 
A.2
B.3
C.6
D.5
Solution
We know that isomers are the molecules with identical molecular formulas but different arrangement of atoms in space. Isomers do not necessarily share similar chemical or physical properties. In many reactions, where products produced are geometrical isomers, then the yield is majority of one isomer and minority of another isomer.
Complete step by step answer:
Isomers are classified
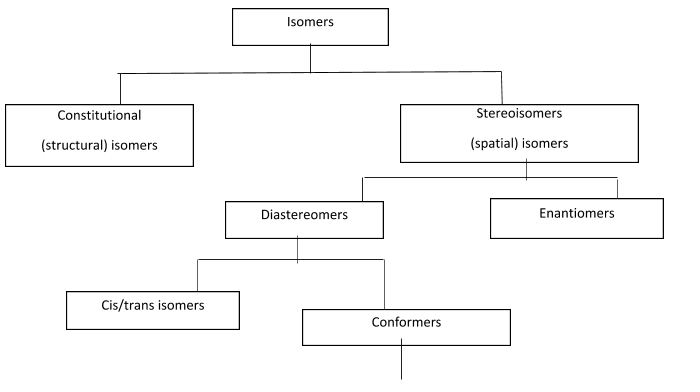
The given compound exhibits structural isomers and is symmetrical. Here, we can see that there are double bonds in both the ends in the long chain of carbon atoms.
We can observe that there are two carbon atoms double bonded, two such structures are bonded with a single bond. There is one alkyl group present on both the structures which is on the upper side of the parent double bonded carbon atoms. And on the other arms only hydrogen atoms are bonded.
The number of sites where geometrical isomers are possible is observed as, n=2.
For symmetrical compounds, we have a predefined formula to calculate the number of geometrical isomers. The equation is given as,
2n−1+2p−1
Where,
p=2n (if n is even)
p=2n+1 (if n is odd)
Here the value of n is even.
Hence, the number of geometrical isomers is =
2n−1+2p−1
On substituting the values we get,
=22−1+222−1
On simplification we get,
=21+20
=2
Hence, the correct answer to the question is option A.
Note:
We have to remember that the isomers have various properties due which they are used for many purposes. Isomers of the same chemical formula can show different physical and chemical properties. Also the same isomer can exist in different excited states that differ by the quantum state of their electrons. Isomers are used for medicinal purposes as well. Enantiomers have a special place in medicinal chemistry as they may possess distinct biological activity.
