Question
Question: How many gauche conformations are possible for n-butane? A. 2 B. 3 C. 4 D. 1...
How many gauche conformations are possible for n-butane?
A. 2
B. 3
C. 4
D. 1
Solution
Conformational isomerism is a kind of stereoisomerism. In confirmation isomerism, the isomers can be inter-convertible by rotations about single bonds. Among the different conformations, Gauche conformation has less steric strain when compared to eclipsed conformation. Less strain means low internal energy and high stability.
Complete step by step solution:
- We have to find how many gauche conformations are possible by n-butane.
- n-butane contains 4 carbon atoms.
- The below representations are called Newman’s conformations of n-butane.
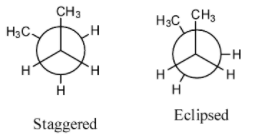
- By rotating one of the carbon atoms by keeping another carbon fixed we will get different conformations of n-butane.
- The following are the possible conformations of n-butane by rotating one of the carbon by keeping another carbon atom fixed.
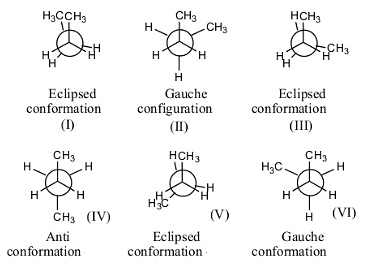
- There are a total of 6 confirmations possible for n-butane.
- Out of those 6, three confirmations are Eclipsed (I, III and V), two confirmations are gauche (II and VI), and one anti conformation (IV).
- Gauche conformation has less strain when compared to eclipsed conformation.
- In eclipsed conformation, the atoms are going to face high repulsion because of the overlapping of one atom with another.
- In n-butane, there are two gauche conformations with less strain.
- Therefore the number of gauche conformations possible for n-butane is 2.
So, the correct option is A.
Note: In Gauche conformation the two atoms or groups have a dihedral angle of more than 0 and less than 120. A conformation in a molecule has one or more gauche connections then it can be called as gauche conformation and gauche conformations are stable than eclipsed conformations.
