Question
Question: How many following oxyacids have peroxy linkage? \({H_2}S{O_3}\),\({H_2}{S_2}{O_8}\),\({H_3}P{O_2}...
How many following oxyacids have peroxy linkage?
H2SO3,H2S2O8,H3PO2,H3PO5,HClO2
Solution
Oxyacids are acidic substances in which one or more hydrogen atoms are bonded to oxygen atoms which are further bonded to other elements subsequently. The compound containing the o-o bond has peroxy linkage.
Complete step by step answer:
We know that oxyacid is known as oxygen containing acid. Oxyacids are prepared by reacting von-metallic acids with water i.e. they react with water to form oxyacids that yield hydronium (H3O+) in solution. The strength of an oxyacid is the determinacy extent to which it dissociates in water. The acid strength can be predicted on the basis of oxidation number and oxidation number. More is electronegativity of an element; more will be its acidic character.
Moreover, as we know prosy linkage is defined as the peroxide linkage or is simply o−o. The single bond present between two oxygen atoms is known as proxy linkage.
Now let us find which of the given compounds has proxy linkage.
(1). H2SO3 This compound is commonly known as sulfurous acid. This hybridization of S is sp3 and its structure is tetrahedral.
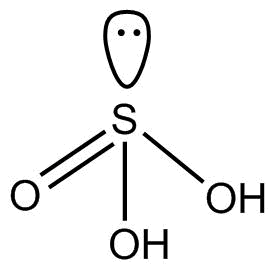
So we can see that there is no proxy linkage in H2SO3.
(2).H2S2O8 This compound is chemically known as peroxydisulfuric acid. The hybridization of each S is sp3 and its structure is as below:-
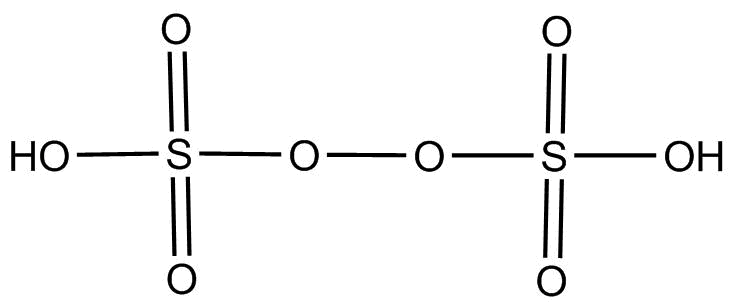
Since o−o linkage is present is its structure. So this compound contains proxy linkage.
(3). H3PO2 This compound is known as hypophosphorous acid and the hybridization of P is sp3 and its structure is:
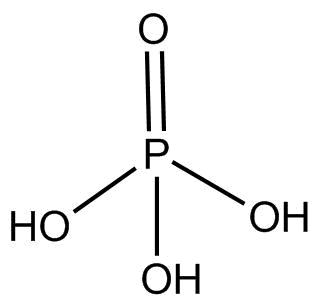
There is no proxy linkage present in this compound.
(4).H3PO5 This compound is known as peroxymonophosplatic acid and its structure is
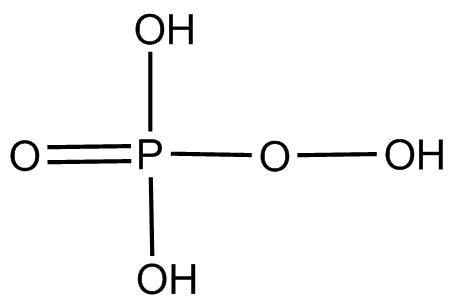
We can clearly see o−o linkage present in the compound. Thus it is oxyacid with proxy linkage.
(5). HCl O2 This compound is known as chorus acid and the hybridization of clatom in the compound is sp3.it is structure is as below
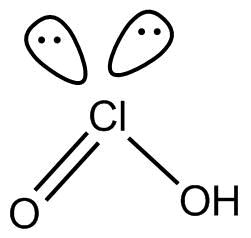
No proxy linkage is present in the compound.
Thus on our observation, we got to know that H2S2O8 and H3PO5 contains proxy linkage.
Note:
The strength of an oxyacid is defined by the extent to which it dissociates in water [i.e. ability to form H+ions]. Oxyacids are mainly used in the synthesis of other chemical compounds and have a wide range of industrial applications.
